On
40
GO
|
Ft3/ft3 of Combustible lb/lb of Combustible —————————————————————————————————— Experimental Error in Heats of Required for Required for Combustion Combustion Flue Products Combustion Flue Products ±% |
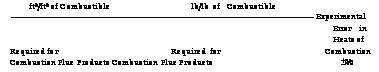 Density Related*5 Heat of Combustion0
Density Related*5 Heat of Combustion0
|
1 |
Carbon |
C |
12.01 |
Gross |
Net*1 |
Gross 14,093e |
Netd 14,093e |
O2 |
N2 |
Air |
Co2 |
H2o |
N2 |
O2 2.664 |
N2 8.863 |
Air 11.527 |
Co2 3.664 |
H2o |
N2 8.863 |
0.012 |
|||
|
2 |
Hydrogen |
H2 |
2.016 |
0.00533 |
187.723 |
0.06959 |
325.0 |
275.0 |
61Д00 |
51,623 |
0.5 |
1.882 |
2.382 |
— |
1.0 |
1.882 |
7.937 |
26.41 |
34.344 |
— |
8.937 |
26.41 |
0.015 |
|
3 |
Oxygen |
O2 |
32.000 |
0.08461 |
11.819 |
1.1053 |
|||||||||||||||||
|
4 |
Nitrogen |
N2 |
28.016 |
0.07439′ |
13.443′ |
0.9718′ |
|||||||||||||||||
|
5 |
Carbon |
CO |
28.01 |
0.07404 |
13.506 |
0.9672 |
321.8 |
321.8 |
4,347 |
4,347 |
0.5 |
1.882 |
2.382 |
1.0 |
— |
1.882 |
0.571 |
1.900 |
2.471 |
1.571 |
— |
1.900 |
0.045 |
|
6 |
Monoxide Carbon |
Co2 |
44.01 |
0.1170 |
8.548 |
1.5282 |
|||||||||||||||||
|
Dioxide Paraffin series CnH2n + 2 7 Methane CH4 |
16.041 |
0.4243 |
23.565 |
0.5543 |
1013.2 |
913.1 |
23,879 |
21,520 |
2.0 |
7.528 |
9.528 |
1.0 |
2.0 |
7.528 |
3.990 |
13.28 |
17.265 |
2.744 |
2.246 |
13.28 |
0.033 |
||
|
8 |
Ethane |
Ca |
30.067 |
0.08029′ |
12.455′ |
0.08029′ |
1792 |
1641 |
22,320 |
20,432 |
3.5 |
13.18 |
16.675 |
2.0 |
3.0 |
13.18 |
3.725 |
12.39 |
16.119 |
2.927 |
1.798 |
12.39 |
0.030 |
|
9 |
Propane |
C3h8 |
44.092 |
0.1196′ |
8.365′ |
0.08029′ |
2590 |
2385 |
21,661 |
19,944 |
5.0 |
18.82 |
23.821 |
3.0 |
4.0 |
18.82 |
3.629 |
12.07 |
15.703 |
2.994 |
1.634 |
12.07 |
0.023 |
|
10 |
«-Butane |
C4H10 |
58.118 |
0.1582′ |
6.321′ |
0.08029′ |
3370 |
3113 |
21,308 |
19,680 |
6.5 |
24.47 |
30.967 |
4.0 |
5.0 |
24.47 |
3.579 |
11.91 |
15.487 |
3.029 |
1.550 |
11.91 |
0.022 |
|
11 |
Isobutane |
C4H10 |
58.118 |
0.1582′ |
6.321′ |
0.08029′ |
3363 |
3105 |
21,257 |
19,629 |
6.5 |
24.47 |
30.967 |
4.0 |
5.0 |
24.47 |
3.579 |
11.91 |
15.487 |
3.029 |
1.550 |
11.91 |
0.019 |
|
12 |
N-Pentane |
C5H12 |
72.144 |
0.1904′ |
5.252′ |
0.08029′ |
4016 |
3709 |
21,091 |
19,517 |
8.0 |
30.11 |
38.114 |
5.0 |
6.0 |
30.11 |
3.548 |
11.81 |
15.353 |
3.050 |
1.498 |
11.81 |
0.025 |
|
13 |
Isopentane |
C5H12 |
72.144 |
0.1904′ |
5.252′ |
0.08029′ |
4008 |
3716 |
21,052 |
19,478 |
8.0 |
30.11 |
38.114 |
5.0 |
6.0 |
30.11 |
3.548 |
11.81 |
15.353 |
3.050 |
1.498 |
11.81 |
0.071 |
|
14 |
Neopentane |
C5H12 |
72.144 |
0.1904′ |
5.252′ |
0.08029′ |
3993 |
3693 |
20,970 |
19,396 |
8.0 |
30.11 |
38.114 |
5.0 |
6.0 |
30.11 |
3.548 |
11.81 |
15.353 |
3.050 |
1.498 |
11.81 |
0.11 |
|
15 |
N-Hexane |
QHM |
86.169 |
0.2274′ |
0.08029′ |
0.08029′ |
4762 |
4412 |
20,940 |
19,403 |
9.5 |
35.76 |
45.260 |
6.0 |
7.0 |
35.76 |
3.528 |
11.74 |
15.266 |
3.064 |
1.464 |
11.74 |
0.05 |
|
Specific Specific Gravity Molecular Density Volume of Air No. Substance Formula Weight3 lb/ft3 ft3/lb = 1.000 Btu/ft3 Btu/lb |
|
Olefin series CnH2n
|
|
Aromatic series CnH2
|
|
Miscellaneous gases
|
|
Appendix B |
![]() Note: All gas volumes corrected to 60°F and 30 in. Hg dry. For gases saturated with water at 60°F/1.73% of the British thermal unit value must be deducted.
Note: All gas volumes corrected to 60°F and 30 in. Hg dry. For gases saturated with water at 60°F/1.73% of the British thermal unit value must be deducted.
A Calculated from atomic weights given in Journal of the American Chemical Society, February 1937.
B Densities calculated from values given in grams per liter at open cycle and 760 mm in the International Critical Tables allowing for the known deviations from the gas laws. Where the coefficient of expansion was not available, the assumed value was taken as 0.0037/°C. Compare this with 0.003662, which is the coefficient for a perfect gas. Where no densities were available, the volume of the mole was taken as 22.4115 L.
C Converted to mean British thermal units per pound (1/180 of the heat per pound of water from 32 to 212°F) from data by Frederick D. Rossini, National Bureau of Standards, letter of April 10, 1937, except as noted.
D Deduction from gross to net heating value determined by deducting 18,919 Btu/lb mol of water in the products of combustion. Osborne, Stimson, and Ginnings, Mechanical Engineering, p. 163, March 1935, and Osborne, Stimson, and Fiock, National Bureau of Standards Research Paper 209.
E National Bureau of Standards, RP 1141.
F Denotes that either the density or the coefficient of expansion has been assumed. Some of the materials cannot exist as gases at 60°F and 30 in. Hg pressure, in which case the values are theoretical ones given for ease of calculation of gas problems. Under the actual concentrations in which these materials are present, their partial pressure is low enough to keep them as gases.
S From third edition of Combustion.
Source: Based on Fuel Flue Gases, 1941 Edition, courtesy of American Gas Association.
[1] The stress values decrease rather rapidly at high temperatures.
• The deterioration is more marked with carbon steel, limiting its usage at elevated temperatures >450°C (~850°F).
• SA 302 is a popular alloy plate material for high-pressure drums due to its sustained high tensility.
• Cr material, 9%, is very well suited for high-temperature tubes and pipes because of its much higher stresses.



 15 сентября, 2013
15 сентября, 2013  admin
admin  Опубликовано в рубрике
Опубликовано в рубрике