Deaeration is the removal of dissolved gases, notably oxygen, from water. Dissolved gases cause many corrosion problems. Pitting caused by O2 (localized corrosion leading to pits) is particularly dangerous because the pits can lead to failure of parts under pressure, even if pitting is not widespread or not much material loss has taken place (Figure 4.1). Ammonia (NH3) readily attacks Cu and Cu-bearing alloys.
Deaeration can be achieved by either mechanical separation or chemical reaction. The practice in the industry is to adopt the following two consecutive steps:
1. Mechanical separation in hot condition, which is very effective, reliable, and cheap
2. Chemical reaction, which is called scavenging, to remove the traces
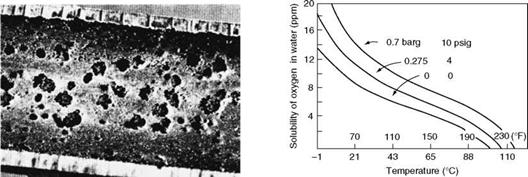
Deaeration is done primarily by heating the incoming water, consisting usually of condensate and makeup (and at times certain waste streams in process plants), by low-pressure steam to its saturation temperature when around 98% of dissolved gases separate from water and vent out. Figure 4.2 gives the solubility of O2 in water. The solubility levels decrease dramatically as the saturation temperature is approached. As even small traces of O2 are exceedingly corrosive to the feed lines and economizer (ECON), a thorough scrubbing of water is necessary to make it completely free of O2. So the deaerators provide a combination of heating and scrubbing and manage to remove all the dissolved O2. Scrubbing action is performed inside a deaerator by any of the following, with progressively increasing scrubbing efficiency and reducing steam consumption:
1. Spray
2. Tray
3. Spray and tray arrangements
In scrubbing action, the following two factors are at work:
1. Water droplets are reduced in size so that the trapped gas has to travel smaller distance to reach the periphery.
2. Surface tension and viscosity are lowered to make it easier for the gas to escape.
|
FIGURE 4.1 Failure due to oxygen pitting. |
![]()
|
FIGURE 4.2 Solubility levels of oxygen in water. |
![]() Steam is the medium for heating the incoming water as it is readily available in a plant, and there is no wastage as the condensed steam is mixed with water. The flow streams of steam and water are in opposite directions in counterflow arrangement for higher heat transfer. Pressure-type deaerators employed in power and process plants deaerate water
Steam is the medium for heating the incoming water as it is readily available in a plant, and there is no wastage as the condensed steam is mixed with water. The flow streams of steam and water are in opposite directions in counterflow arrangement for higher heat transfer. Pressure-type deaerators employed in power and process plants deaerate water
|
FIGURE 4.3 Spray — and tray-type vertical deaerator without feed tank. |
To the extent of <0.005 cc/L or 0.007 ppm (7 ppb) of O2 with steam vent loss of <1% inlet steam by weight. A healthy tall plume of ~1 m of steam and air from the vent will ensure proper removal of oxygen from a deaerator.
Deaerators can be vertical or horizontal to suit the space and head room. They are mounted on feed tanks, which provide storage as desired, 10-20 min being normal in a power plant.
The shell is made of carbon steel (CS), but the internals that come in contact with gases are of stainless steel (ss), to withstand the corrosion of oxygen. The spring-loaded nozzles of a deaerator are the heart of the system, as they provide the desired shape of spray with required fineness of particles. They are the proprietary items of a manufacturer.
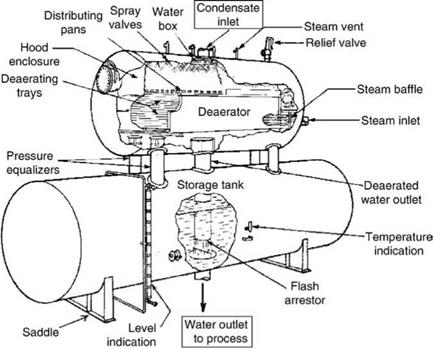
Figures 4.3 and 4.4 show the two types of deaerators—vertical and horizontal—both having spray and tray arrangements for high efficiency and reduced steam consumption. The vertical deaerator here is shown without the tank.
A typical deaerator is shown in Figure 4.5. The deaerators have mainly two limitations:
1. They can remove many dissolved gases of the condensate but not the large quantities of dissolved gases found in the waste streams of many industrial plants.
2. Although free CO2 is removed entirely, only a small part of the combined CO2 is released in the deaerator and the bulk is released with the steam in the boiler. Thereafter it is dissolved in the condensate, causing corrosion. This can be controlled by using neutralizing or filming amines.
|
FIGURE 4.4 Spray — and tray-type horizontal deaerator mounted on feed tank. |
|
Condensate Return |
![]()
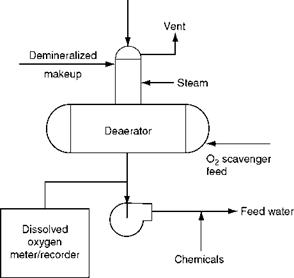 FIGURE 4.5
FIGURE 4.5
Schematic arrangement of a deaerator.
Removal of last traces of oxygen is done by chemical scavengers such as sodium sulfite (Na2SO3) or hydrazine (N2H4).
For boilers operating at pressures <70 bar, catalyzed Na2SO3 is the most common O2 scavenger due to its following features:
1. Low cost
2. Ease of handling
3. Nonscaling properties
• Na2SO3 with or without the catalyst is an efficient and fast O2 scavenger even at low temperatures. But at higher temperatures like 100°C, the reaction is really rapid. For every rise of 10°C the speed of reaction doubles.
• The reaction proceeds rapidly at pH values between 9 and 10.
• Na2SO3 added to the solids in boiler water increases the carryover, unlike hydrazine, which turns eventually into N2 and H2O. This addition is unsuitable where spray attemperation is to be done on steam unless it can be fed beyond the point from which FW for desuperheating is taken.
• Theoretically, 7.88 ppm of pure Na2SO3 is required for each ppm of dissolved O2. But for technical-grade catalyzed Na2SO3, it is appropriate to consider 10 ppm or 10 kg/1 kg of O2 present in FW.
• Na2SO3 should be dosed only on a continuous basis to achieve complete O2 removal. Intermittent feeding is not recommended except for low-pressure systems.
• Fe, Cu, Co, Ni, and Mn are among the most effective materials for acting as catalysts for Na2SO3. Typically catalyzed Na2SO3 can reduce O2 nearly completely in 10 s whereas plain Na2SO3 can take even 10 min to reduce O2 from 9.8 to 6.6 ppm.
• Where ECONs are used, sulfite residuals of 10-15 ppm with pH > 8.3 are recommended for protection against O2 attack.
For boilers operating at pressures >70 bar, hydrazine is preferred to sulfite as
1. Hydrazine adds no solids to the boiler water.
2. Na2SO3 can decompose at higher pressures to form H2S and SO2 that can cause
Corrosion of return condensate system.
As pure hydrazine has low flash point, a 35% solution is used. Theoretically, 1 ppm of hydrazine is required to remove 1 ppm of dissolved O2 but in reality is between 1 and 1.5 ppm.
• With higher water temperatures and pressures, hydrazine is preferred to Na2SO3
Although it is much slower because it adds no solids to boiler water. Hence it is well
Suited to spray attemperator application.
• An added advantage is its ability to passivate Fe — and Cu-bearing surfaces.
|
Time (min) |
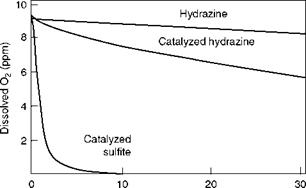
FIGURE 4.6
Effectiveness of O2 scavengers.
• Organically catalyzed NH4, with reaction times speeded 10 to 100 times and with passivating properties also enhanced, is in a position to extend application to a medium pressure of 45 bar.
• Concern about cancer-producing properties dictates extremely careful handling.
• Another concern is the breakdown of hydrazine into ammonia, which is highly corrosive to Cu and Cu-bearing alloys, and a very careful control of the dosage is required.
• Reaction with O2 depends on the water temperature, pH, and impurities. Figure 4.6 compares the speeds of reaction of Na2SO3 and N2H4 and the effect of catalyzed N2H4.
Substitutes for O2 scavengers available in the market must be examined closely before using.



 22 августа, 2013
22 августа, 2013  admin
admin 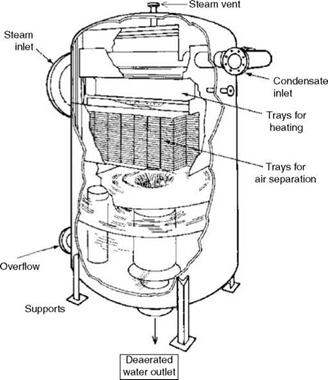
 Опубликовано в рубрике
Опубликовано в рубрике