Carryover from a drum is the entrainment of contaminants in steam—solid, liquid, or vapors. It consists mostly of boiler water droplets with solids—both suspended and dissolved. Silica is carried over in vaporous form. It is necessary to distinguish between steam purity and quality:
• Purity is the amount of contaminant leaving the drum, measured in parts per million.
• Quality is the amount of moisture in steam, measured as dryness.
Although the contaminants are in all three phases, steam purity is reported as the carryover of solids. This is because the solids are the most troublesome due to their scale-forming tendency. Solid and liquid impurities are, therefore, water droplets containing mostly Na salts with minor amounts of Ca, Mg, Fe, and Cu. Gaseous constituents found in steam at <130 bar pressure are NH3, N2H4, CO2, N2, SO2, SiO2, and amines, of which only SiO2 is troublesome. The others only interfere with steam purity measurement.
Carryover of solids results in deposit formation in SH, stop and control valves, and turbine. It can also contaminate the process streams and spoil the product quality. SH deposits cause tube overheating and lead to failure. Deposits in turbine control valves can cause turbine overspeeding, which is dangerous. Turbine efficiencies can drop by as much as 5% due to the scaling on the blades.
Low-pressure process steam can accept dry steam, and 99% dryness or steam quality is the norm if there is no need to feed a turbine. Decades ago, this was the industry standard until the concept changed from steam quality to steam purity at the initiative of the turbine makers, as better performance and lower turbine maintenance could be assured. The steam purity limit was fixed at 1 ppm maximum. This is still the norm as the boilermakers find it difficult to execute guarantees with the commercial instrumentation at site, although boilers with well-designed and proven drum internals routinely deliver well below 0.1 ppm of solids. But in modern high-efficiency utility turbines, with much closer tolerances and longer intervals for overhauls, 1 ppm guarantee is found to be unaccept — ably high. Various utilities and turbine makers demand different purity levels, sometimes almost zero solids, which is impractical. However, there is a consensus with regard to the main offender, namely, silica, the requirement of which is restricted to 0.02 ppm or 20 ppb. As steam expands in the turbine and approaches the saturation point, silica tends to separate out and deposit on the blades. These insoluble deposits on the turbine blades in the medium — and low-pressure zones start affecting the output and, at times, cause vibrations, requiring costly outages for total dismantling and cleaning. At <20 ppb, these deposits could be avoided. The current steam purity standards, though varying somewhat, are built around purity levels that would ultimately deliver silica of <20 ppb. This, together with 0.03 ppm or 30 ppb of solids in steam, is acceptable in most applications to ensure uninterrupted service from SH and turbine.
Carryover is the result of mechanical, chemical, design, and operational factors:
1. Mechanical. Minute inefficiency of steam separators, which is inevitable, leakage in separators, high water level, and so on
2. Chemical. High solid concentration, excessive alkalinity, presence of oil and other organic contaminants, and the property of silica to evaporate at high pressures
3. Design. Higher loading of the separators and greater speed of separation at the steam-water interface
4. Operational. High water levels in the drum caused by foaming and priming
Steam separation in a modern water tube boiler, from a large body of vigorously circulating steam-water mixture (ranging over 5-50 times of the steam output or higher, depending on the boiler pressure) in the confines of a small drum, is a complicated process. The efficiency of separation under steady-state conditions is >99.999%. The turbulence in the drum can throw up slugs of water into the steam space causing priming, because of sudden pressure and flow caused by disturbances, leading to a rise in water levels in the drum.
Steam separation from drum water is effected in two stages.
1. The primary separation is either by centrifugal action as in vertical (Figure 4.10) or horizontal cyclones or by inertial action as in deflection baffles (Figure 4.11), where steam is separated from a large body of water. A large density difference (115 at 15 bar and 20 at 70 bar) coupled with abrupt changes of direction in inertial separators or violent centrifugal action in cyclones separates steam from water effectively.
2. The secondary separation, called steam scrubbing or steam drying, involves separation of small traces of water from large amounts of steam. This is accomplished by providing a large contact surface with flow changes or interceptions as in corrugated baffle plates or demisters. The action is steam adsorption due to its affinity to steel. Velocities are kept low to help steam adsorption and prevent re-entrainment.
The deflection baffle plate for guiding the steam from riser tubes to the primary steam separators and the secondary scrubbers are all of bolted construction to permit the periodic inspection of the internal surface of the drum. It is of utmost importance that this assembly is manufactured and installed with great care to prevent any leakage of drum water into steam space.
|
FIGURE 4.10 Centrifugal steam separation. |
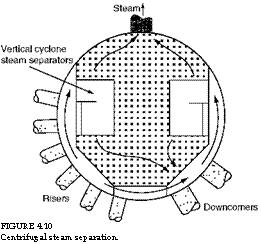 This two-stage steam separation is very effective. So, when higher purity levels are desired, the solid contents of drum water are reduced.
This two-stage steam separation is very effective. So, when higher purity levels are desired, the solid contents of drum water are reduced.
|
FIGURE 4.11 Inertial steam separation. |
|
Drum Pressure |
Silica |
|
|
In barg |
In ppm |
|
|
In psig |
(approximate) |
(maximum) |
|
<300 |
<20 |
150 |
|
301-450 |
21-30 |
90 |
|
451-600 |
31-40 |
40 |
|
601-750 |
41-50 |
30 |
|
751-900 |
51-60 |
20 |
|
901-1000 |
61-70 |
8 |
|
1000-1500 |
71-100 |
2 |
|
1501-2000 |
101-140 |
1 |
Careful placement of drum internal pipes for dosing, blowdown, and FW distribution is very important to avoid disturbance to the water levels, which can provoke carryover. Drum sizing is important because it can accommodate all the separators and provide adequate volume for reasonable water level fluctuations inevitable in plant operation.
The reader can refer to Section 7.2.1.2 on boiler fabricated parts for additional description on steam separators.
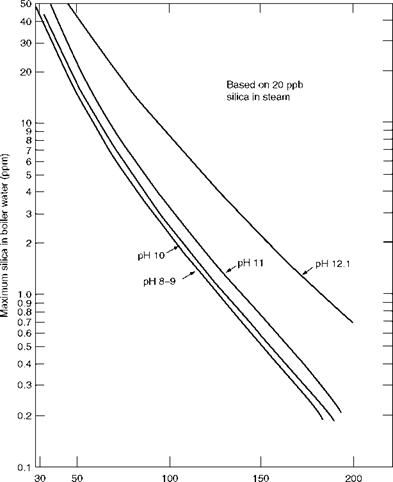
At pressures >80 bar, silica has a tendency to evaporate with steam, thus escaping its capture in steam separators. Selective vaporous carryover of silica can occur at a boiler pressure as low as 30 bar. The only way is to reduce the silica content in drum water. Accordingly, at high pressures, monitoring silica level gains precedence over TDS. Recommended levels of silica in boiler water to obtain 15-20 ppb of silica concentration in steam as per ASME 1975 are given in Table 4.5. Figure 4.12 captures the pH values of the boiler water for higher pressures up to 200 bar.
Steam separators in boiler circulation systems provide water-free steam to SH and steam — free water to downcomer circuits. At higher boiler pressures, the amount of water in circulation reduces. Sizing of drum cyclones is governed by the capacity of
• Water flow at low pressures
• Steam flow at higher pressures
Steam release rates per meter length of drum increase at higher pressures and can vary from 6 tph/m at 10 bar to 20 tph/m at 70 bar and even 60 tph/m at 200 bar for the same diameter of drum depending on the fuel, boiler pressure, type of boiler, and number of separators fitted inside. For pressures >130 bar, the sizing of steam separators is very critical to ensure that no overloading takes place, which can result in higher carryover.
|
Boiler pressure (bar) FIGURE 4.12 Permissible concentrations of silica in boiler water at various pressures and pH values. |
Foaming
Steam bubbles routinely keep bursting at the steam-water interface in the drum, causing small water droplets to get entrained into the steam. This interface is the normal water level in the drum or the interface in the steam separators. As the steaming rate increases, a point is reached when the steam bubbles reaching the surface are more than the bubbles bursting, which results in foaming, usually caused when the limits in the drum are exceeded for
• Total and suspended solids
• Alkalinity
• Oil
• Phosphates
Foaming results in an increased carryover as the steam moving toward the outlet tends to carry more particles, since the water level is higher due to foam. Further, as the water level along the drum length is uneven, depending on the locations of level indicators and controls, incorrect feeding of water can aggravate the problem by further increasing the carryover. It is interesting to note that the factors mentioned earlier have been found in the boilers experiencing foaming, but on many occasions, foaming has not taken place in spite of their presence.
Priming is a condition in which slugs of water enter from the drum to the steam outlet due to a combination of:
• Sudden lowering of drum pressure
• Increase in steaming rate
• Foaming tendency
Another factor may be spouting of submerged riser pipe. The water level is raised and the steam space is reduced, leading to the cloud of water droplets and foam to be raised close to the steam outlet. In such conditions, priming occurs and there is excessive moisture in the steam because of the spouting or surging of boiler water. Note: Priming is not caused by boiler water conditions but by increased water level. Operating the boiler properly with the specified feed and boiler water conditions is a way to eliminate priming and foaming problems.
There are basically two types of steam purity measurements in commercial practice, both amenable to on-load continuous measurements by which peaks and surges can be detected and recorded.
1. Conductivity method for purity levels >0.5 ppm of solids
2. Sodium flame photometer for very accurate measurements of total solids at levels as low as 0.001 ppm or 1 ppb
Steam sampling has to be treated as an integral part of steam purity measurement and must be carefully carried out to derive correct results.
• Sampling lines have to be small bore pipes as short as possible.
• They should preferably be made of ss to prevent the addition of any corrosion products to the sample.
• Reference should be made to ASME PTC 19.11-1997—Steam and water sampling, conditioning and analysis in the power cycle, as it describes the sampling and measurement procedures in proper detail.
Pure steam and water are nonconductors of electricity. As the impurities increase, the conductivity increases. One part per million of solids impurities in steam corresponds to a conductivity of —0.55 ^S/cm (1 ppm = 2 ^S/cm). Commercial purity levels of <1 ppm are very well addressed by this simple, inexpensive, and online measuring system. The only precaution required is to avoid the ingress of dissolved gases, particularly CO2 and NH3, which interfere with the reading. Degassing devices are available. Per ppm of NH3 and CO2, the conductivity reading is approximately 8 and 5, respectively, much higher than it is for dissolved solids (between 1 and 2), thereby materially affecting the readings. To correct the readings, no direct measurement of individual constituents is possible. But the data can be obtained indirectly by analyzing the boiler water for various constituents and apportioning the solids in steam in the same ratio. The impurities in steam are derived entirely from the boiler water only.
Traces of Na are present in boiler water and in the condensed steam samples in the same proportion as their respective dissolved solids. By measuring them in a photometer and analyzing the boiler water for the solids, it is possible to determine accurately the solids in the steam. Accuracy levels of 6 ppb of solids are claimed by suppliers for portable units that can be used on site. Each 0.1 ppm of Na in steam contains -0.3 ppm of solids.
Gravimetric methods measure purity by carefully collecting a large amount of condensed sample of steam, evaporating it to dryness, and measuring the residue. This is slow, requires a careful handling, and does not lend itself to routine or online measurement.
Another outdated method for dryness of steam is the throttling calorimeter. A sample of saturated steam is expanded through a short orifice into atmospheric pressure and the temperature is measured. The difference between the theoretical and the actual superheats represents the moisture in steam evaporated. Solid content in steam is deduced from the solids in the boiler water. The accuracy is only ±2% moisture that can work out to as high as ±7 ppm of solids.
Further Readings
ASME PTC 19.11-1997—Steam and water sampling, conditioning, and analysis in the power cycle.
Betz, 1991, Handbook of Industrial Water Conditioning, 9th Edition.
BS 2486, 1997, Recommendations for treatment of water for steam boilers and water heaters.
Cotton, I. J., 1980, Optimise oxygen control in your boiler feed system, Betz Laboratories Inc., Power, October.
Powell, T. S, 1954, Water Conditioning for Industry, Mc-Graw Hill, London.
Strauss, D. S., 1986, Advances in chemical water treatment improve reliability of steam-generating systems, Power, October.
Strauss, D. S., Keen, S., and Puckorius, P., 1987, Boiler water treatment for low and moderate-pressure plants, Power, Special Report, June.
Water Treatment for Industrial Boilers by Babcock and Wilcox Co., Power Generation Division.



 22 августа, 2013
22 августа, 2013  admin
admin 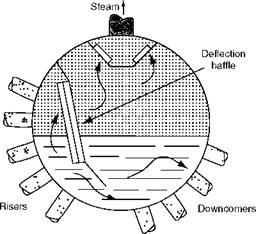
 Опубликовано в рубрике
Опубликовано в рубрике