Combustion is the self-sustaining rapid exothermic chemical reaction between the elemental C, H, and S of fuel with O2 from air due to their affinity. The products of combustion are CO2, H2O, and SO2 along with heat. C and H are beneficial constituents of fuel, producing heat, whereas S is the least heat generating and most harmful, as the SO2 produced causes low-temperature corrosion. To make any combustion self-sustaining, the resultant temperature from the exothermic reaction should exceed the ignition temperature of the fuel.
Every combustion process proceeds in three stages.
1. Drying. The moisture in the fuel is evaporated rapidly by flash drying, mainly by the hot air and furnace radiation.
2. Burning of VM. VM leaves the fuel progressively due to the radiation from the furnace and hot furnace gases and burns in furnace space.
3. Burning of FC. The dry and devolatilized fuel burns somewhat slowly, releasing the maximum heat on the grate in stoker firing or in the bed in FBC or in the furnace in PF or oil — or gas-fired boilers.
After flash drying of fuel, for the actual combustion to start, necessary air for combustion and heat energy for ignition are required to enable the fuel reach a certain temperature called the ignition temperature, the minimum temperature at which the combustibles can react with O2. The ignition temperature of fixed carbon can be considered as the ignition temperature of fuel as the volatiles leave the bed quickly. The ignition temperatures vary widely (please refer to Table 3.2 and Figure 3.5.).
O2 is the sole supporter of combustion in boiler furnaces. A combustible substance is capable of reacting with O2 to produce heat. The affinity of a combustible to O2 governs the speed of combustion. This speed of O2 reaction can vary enormously—very slow as in the case of rust formation or very instantaneous as in the case of gas firing. Combustion is complete when all combustibles have reacted with O2 to the full extent to which they are capable of reacting. This is important to note, as C can react to form both CO and CO2, but the combustion is complete only when all C is converted into CO2. Table 2.12 lists combustibles encountered in boiler furnaces.
Atmospheric air is a mixture (not a compound) of mainly N2 and O2 in the ratio of 79:21 by volume and 77:23 by weight. It also contains small amounts of water vapor, CO2, and other inert gases such as argon, but for engineering calculation purposes they are ignored. N2 reported earlier is inclusive of CO2 and inert gases.
Dry air together with the associated water vapor becomes wet air, which is found in the atmosphere. A psychometric chart gives the water content in air at various temperatures and relative humidity. Dry air has
• An apparent molecular weight (MW) of 29 kg/kg mol
• Density of 1.29 kg/m3 or 0.0805 lb/ft3 at NTP conditions (760 mmHg, 0°C)
• Specific volume of 0.7728 m3/kg or 12.38 ft3/lb at NTP conditions
• Relative density (w. r.t. H = 1) of 14.38
Dry air/unit O2 is 4.32 kg/kg. All combustion calculations are performed on a dry air basis, and humidity is added separately to arrive at the wet air (Figure 2.34).
Stoichiometric air is the amount of theoretical air required for the combustion of fuel and can be derived from the fuel composition. In actual practice a certain amount of excess air is needed to complete the combustion, depending on the fuel, firing equipment, combustion temperature, etc. Table 2.13 shows the range of excess air needed for optimum combustion
|
Combustible |
Symbol |
Molecular Weight |
Combustion Reaction |
Heat (GCV) (kcal/kg) |
Heat (GCV) (Btu/lb) |
|
Carbon |
C |
12 |
C + O2 = CO2 |
7,833 |
14,122 |
|
Hydrogen |
H2 |
2 |
H2 + 0 = H2O |
33,945 |
61,122 |
|
Sulfur |
S |
32 |
S +2 O2 = SO22 |
2,212 |
3,983 |
|
Hydrogen sulfide |
H2S |
34 |
H2S + 1.502 = S02 + H20 |
3,944 |
7,122 |
|
Methane |
CH4 |
16 |
CH4 + 202 = C02 + 2H20 |
13,266 |
23,879 |
|
Ethane |
C2H6 |
30 |
C2H6 + 3O2 = 2C02 + 3H20 |
12,422 |
22,322 |
|
Propane |
CsH8 |
44 |
C3H8 + 4H20 = 3C02 + 4H20 |
12,033 |
21,662 |
|
Butane |
C4H10 |
58 |
C4H10 + 5H20 = 4C02 + 5H20 |
11,838 |
21,328 |
|
Pentane |
C5H12 |
72 |
C5^12 + 602 = 5C02 + 6H2O |
11,717 |
21,291 |
|
Note: Refer to Appendix B for a comprehensive list of combustibles and their heats of combustion. |
|
0.010 0.008 0.006 0.004 0.002 20 25 30 35 Dry bulb temperature (°C) FIGURE 2.34 Psychrometric chart in SI units. |
For various fuels in boiler practice. Also refer to Section 10.2.4 for additional discussion on excess air.
|
Normal Excess Air Percents for Various Fuels at Full Load |
![]() Fuel-air reactions are exothermic and hence produce heat. Various constituents of fuel react with air to produce different amounts of heat. Table 2.12 gives the combustion reaction and heat produced for various combustibles. The higher heating values or gross
Fuel-air reactions are exothermic and hence produce heat. Various constituents of fuel react with air to produce different amounts of heat. Table 2.12 gives the combustion reaction and heat produced for various combustibles. The higher heating values or gross
calorific values are listed in Table 2.12. These values are measured by bomb calorimeter for solid and liquid fuels in which the combustible substances are burned to completion at a constant volume. Calorimeters of continuous or constant flow are used for gaseous fuels. Tables 2.14 and 2.15 list the combustion data for select gases on both weight and volume bases, respectively. Also refer to Appendix B for a detailed chart.
|
Fuel |
Firing |
Excess Air by Weight at Full Load (%) |
|
Pulverized coal |
Water-cooled furnace |
15-20 |
|
Coal |
Stoker |
30-45 |
|
Fuel oil |
Register burner |
3-15 |
|
NG, RG, COG |
Register burner |
3-10 |
|
BFG |
Scroll |
15-20 |
|
Bagasse |
All |
25-35 |
|
Black Liquor |
Recovery furnace |
5-7 |
|
TABLE 2.13 |
|
TABLE 2.14 Combustion Data on Weight Basis (kg/kg)
|
|
TABLE 2.15 Combustion Data of Gases on m3/m3 Basis |
|||||||
|
Combustibles |
Symbol |
Mol. Wt. |
Required (m3/m3) |
Products of Combustion (m3/m3) |
|||
|
O2 |
Air |
CO2 |
H2O |
N2 CO SO2 |
|||
|
Carbon monoxide |
C0 |
28 |
0.5 |
2.380 |
1 |
1.880 |
|
|
Hydrogen |
H2 |
2 |
0.5 |
2.380 |
1 |
1.880 |
|
|
Hydrogen sulfide |
H2S |
34 |
1.5 |
7.150 |
1 |
5.646 1 |
|
|
Methane |
CH4 |
16 |
2.0 |
9.530 |
1 |
2 |
7.530 |
|
Ethane |
C2H6 |
30 |
3.5 |
16.675 |
2 |
3 |
13.170 |
|
Propane |
C3H8 |
44 |
5.0 |
23.820 |
3 |
4 |
18.820 |
|
Butane |
C4H10 |
58 |
6.5 |
30.970 |
4 |
5 |
24.470 |
|
Pentane |
C5H12 |
72 |
8.0 |
38.110 |
5 |
6 |
30.110 |
Three Ts
Time, temperature, and turbulence are the three classical Ts that are required for completeness of combustion.
• Time. Adequate time is needed for completing the combustion reaction in the form of residence time in furnace and traveling time in stoker.
• Temperature. Minimum ignition temperature has to be reached for initiation of combustion. Higher temperatures of combustion air increase the speed of combustion. Hot air is at times essential, especially for high moisture fuels, to speed the combustion reaction.
• Turbulence. An intimate contact of fuel with air is essential. A vigorous turbulence helps mixing and scrubbing of fuel of the ash formed on surface.
This is the theoretical temperature that a combustion reaction can produce with complete combustion taking place at stoichiometric condition, assuming that there is no loss of heat to the surroundings and no dissociation. In practice, however, the actual flame temperature will always be lower than the adiabatic temperature, depending on excess air, water cooling and the extent of dissociation.
Dissociation is the breakdown of some CO2 and H2O into elements, which is an endo — thermic reaction occurring at 1650°C and above. Typically —10% of CO2 and 3% H2O can break down, absorbing 2,414 and 33,945 kcal/kg (4,345 and 61,100 Btu/lb) of heat and lowering the flame temperature. It must be remembered that at lower temperatures the gases recombine and generate heat. There is, thus, no loss of heat but only the lowering of flame temperature.
The adiabatic temperature can only be calculated by dividing the total heat by the weight of combustion gases (where the total heat is the heat of combustion + heat of air and fuel) and using the gas tables to arrive at the temperature.
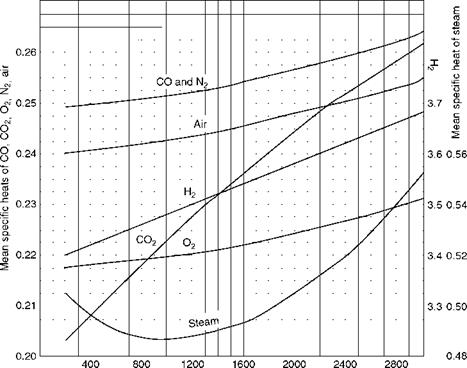
Specific heats of gas are required to construct the enthalpy-temperature relationships of gas tables. Specific heat is the heat required to raise the unit weight of substance through unit temperature—kilocalories per kilogram degree Celsius or British thermal units per pound degree Fahrenheit. As all substances vary in volume and pressure with variation in temperature, the specific heat can be at either constant volume or at constant pressure. In liquids and solids there is negligible variation when they are heated or cooled; in gases the change in pressure and volume is sizable. Therefore, there is no difference in the specific heats for solids and liquids, whereas for gases the specific heats are different at constant pressure Cp or at constant volume Cv. Always Cp is higher than Cv by the amount of heat required to do the work of expansion. Figure 2.35 illustrates the mean Cp values at a constant atmospheric pressure for various gases and steam. Instantaneous values will be different from these. For monatomic gases, k value, which is Cp/Cv, is 1.66, and for diatomic gases k is 1.4.
Combustion air and gas weight estimation from fuel analysis is the first requirement in boiler design or fan sizing.
|
Temperature (°C) 205 427 650 870 1093 1316 1538
Temperature (°F) FIGURE 2.35 Approximate mean specific heats of gases and steam. |
Conversion of Proximate to Ultimate Fuel Analysis for Coals
Proximate fuel analysis (PA) and ultimate fuel analysis (UA) are two ways of expressing the fuel constituents, each with a different purpose as explained in Chapter 1. Ultimate
Analysis is amenable to calculations using the combustion constants, whereas the PA is
Better suited for graphical solution. It is possible to arrive at a fairly accurate UA from PA using empirical methods. Gebhardt’s method is a popular one where the daf % VM and FC of PA are converted into the daf % of C, H, N, and O in UA as shown:
• % daf C = FC + 0.02 VM2 for anthracite
= FC + 0.9 (VM-10) for semianthracite
= FC + 0.9 (VM-14) for bituminous coal
= FC + 0.9 (VM-18) for lignites
• % daf H = VM [(7.35/VM + 10) — 0.013]
• % daf N = 0.07 VM for anthracite and semianthracite
= 2.1-0.012 VM for bituminous coals and lignites
• % daf O = 100 — (C+H+N)
Fixed carbon and VM are on a daf basis and %C derived includes the %S.
Air and Gas Weights from Ultimate Analysis
• From the UA, air and gas weights can be calculated using the table of gas constants or Equation (2.31) to arrive at the dry weights.
• Theoretical dry air for combustion is given as
|
|
|
Kg of air/kg of fuel fired |
(2.31)
Where C, H, O, and S are percent by weight in UA.
• Humidity in air at ambient conditions can be read from a psychometric chart and added to arrive at the wet air.
• For gas weight on wet basis the weight of actual fuel burnt, that is, fuel weight-ash weight, has to be added to the weight of wet air.
An actual worked-out example for estimating the air and gas weights from UA is provided in Appendix A.
Air and Gas Weights from Proximate Analysis
For Solid Fuels: Graphical Method
When air and gas weights are to be evolved from PA
• UA can be first calculated from PA and the aforementioned method can be used or
• A graphical solution can be adopted
Both methods give sufficiently accurate results for commercial purposes and vary from
1 to 2%.
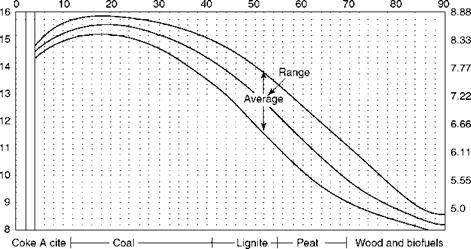
The graphical method for solid fuels with PA employs Seyler’s chart, which is described in Chapter 3 and daf VM is the base. The range of VM for various fuels is indicated in Figure 2.36. The figure covers a wide range of solid fuels and provides all the necessary combustion data.
• From the PA, daf VM is obtained by dividing actual VM by (1-[ash + moisture]).
• Theoretical combustion air for daf GCV in terms of kilograms per 1000 kilocalories cals per kilogram can be read from Figure 2.36. Actual air is then obtained by multiplying the GCV.
• H2 on a daf basis is shown in Table 2.16. This is to be multiplied by (1-[ash + moisture]) to obtain the actual H2. Nine times H2 gives the moisture on combustion.
• The actual CO2 by volume% is equal to
|
(2.32) |
![]() Theoretical maximum CO2 by volume
Theoretical maximum CO2 by volume
1 + f (excess air%/100)
Which gives a relationship of CO2 to excess air. Theoretical CO2 and factor f are listed in Table 2.16.
|
TABLE 2.16 Combustion Parameters for Solid Fuels Coke and Anthracite Coal Lignite
|
|
M O O O |
![]()
|
O > O O "ft ■O |
![]()
|
CL F G O < O |
![]()
|
O O O |
![]()
|
9L K CQ |
![]()
|
CЫ O O O 0~ |
![]()
|
Daf VM % in fuel |
![]()
|
FIGURE 2.36 Combustion properties of solid fuels. |
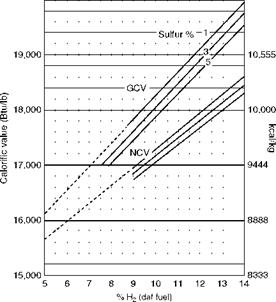
![]() Air and Gas Weights from Proximate Analysis for Liquid Fuels—Graphical Method
Air and Gas Weights from Proximate Analysis for Liquid Fuels—Graphical Method
This is a quick and reliable method for calculating the air quantity and CO2% by volume.
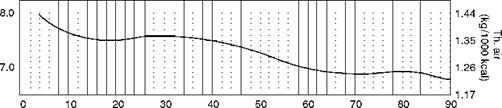
• The kilocalorie method for liquid fuels uses daf H2 in fuel as the base.
• For each 1% increase in sulfur, the CV has to be reduced by 55 kcal/kg or 100 Btu/lb.
• Normally, higher sulfur is associated with higher hydrogen.
At lower levels of H2 the CV values are not very accurate and hence shown as dotted lines (Figure 2.37). However, CO2 values are not affected.
This is not applicable for vegetable oils or manufactured oils, which are generally high in O2 content.
|
I and natural gas, the data of Table 2.17 |
![]() Typically for fuel oil (with moderate sulfur of can be applied.
Typically for fuel oil (with moderate sulfur of can be applied.
|
|
|
CO 1.35 10 1.33 00 |
|
1.31 k 0 1 |
|
CЫ. |
![]()
|
F 1.10 5 1.05 1.00 7.5 И ° 7.4 Ј 7.3 |
![]() FIGURE 2.37
FIGURE 2.37
Fuel oil combustion properties.
|
TABLE 2.17 Air Requirement for Typical Fuel Oil and Natural Gas
|
This is continuously monitored in a boiler as it is the only way to measure the completeness of combustion. Commercial control room indication is for O2, CO2, and CO by percentage of volume on dry basis.
• Adequate O2 reading on panel assures that there is excess air.
• Little or no CO indicates a good mixing of air and fuel leading to complete combustion.
• O2 or CO2 is measured by the gauge instrumentation. O2 measurement is generally preferred due to ease and consistency of measurement and directness of result.
• For testing purposes, CO2 measurement is mandatory and is usually performed by Orsat analyzer.
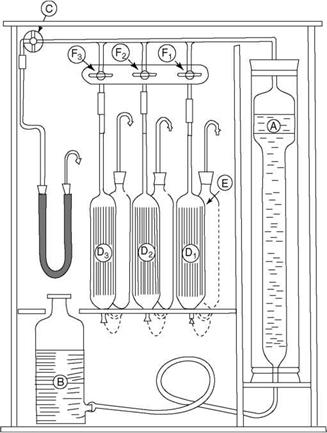
Orsat analysis is still considered the best method to analyze the flue gases accurately. Orsat apparatus (Figure 2.38) consists of (A) a burette graduated bottom upward into
|
|
FIGURE 2.38
Orsat analyzer.
100 divisions, (B) an aspirator bottle, and (Dj-D3) three pipettes containing absorbents filled with glass tubes or beads (for increasing the area of absorption).
• Potassium hydroxide solution (KOH) for CO2 (and also SO2)
• Freshly prepared alkaline pyrogallol or pyrogallic acid for O2
• A solution of cuprous chloride (CuCl2) in NH3 or HCl for CO (and also for ethylene and acetylene)
Each pipette is connected to an empty pipette behind it so that the absorbent can recede into it as the gas is admitted. There is (C) the main isolating cock for gas retention in the system along with (F1-F3) the three isolating cocks for the three pipettes.
The procedure consists of (1) aspirating 100 cc of gas with the help of the aspirator bottle, (2) absorbing the gas in the three absorbents in a definite sequence, and (3) noting the absorption by difference in the measuring burette. Care, strict adherence to the procedure, and the correct preparation of absorbents to specified concentration are needed to obtain error-free results. For getting proper samples on a sustained basis, a suction pump is used for sucking flue gas, as the aspirator alone is usually not adequate.
Some Graphical Solutions for Flue Gas
Weight of dry flue gas per kilogram of fuel from flue gas analysis is given by
11CO2 + 7CO + 7N2 + 8O, (_ S ^
——— 23(CO2 + CO)2 2 x I Cg + 183 J (2-33)
Where
CO2, CO, N2, and O2 = gas volumes in percentage in dry flue gases
S = percentage by weight of sulfur in fuel
Cg = weight of carbon in flue gas per kilogram of fuel
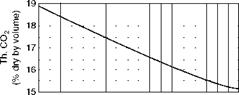
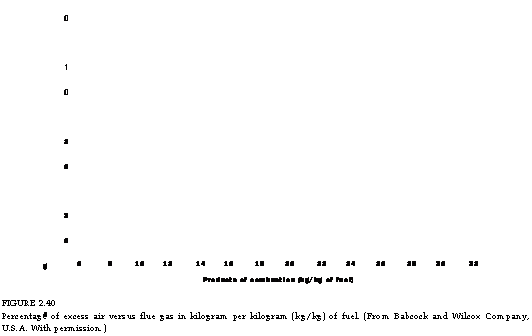
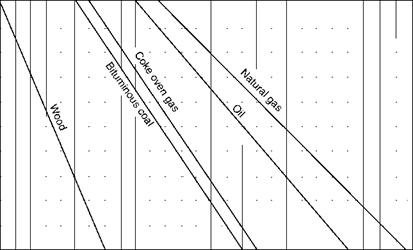
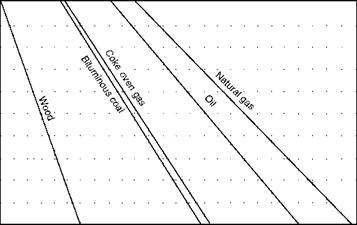
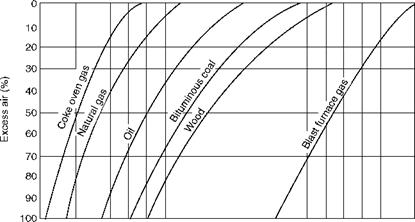
Figures 2.39 through 2.41, illustrate CO2% in flue gas, weight of flue gas, and combustion air for various percentages of excess air for a variety of popular fuels.
|
4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 CO2 (percent by volume in flue gases) FIGURE 2.39 Percentage of CO2 in dry flue gas versus excess air. (From Babcock and Wilcox Company, U. S.A. With permission.) |
|
0 G 40 “ 50 W I§ 60 80 90 100 |
|
6 8 10 12 14 16 18 20 22 24 26 28 30 32 Products of combustion (kg/kg of fuel) |
|
FIGURE 2.40 Percentage of excess air versus flue gas in kilogram per kilogram (kg/kg) of fuel. (From Babcock and Wilcox Company, U. S.A. With permission.) |
|
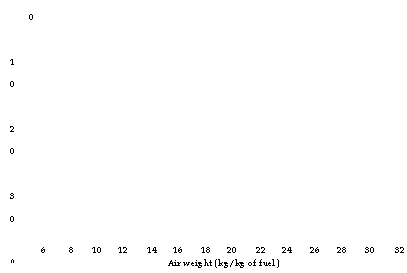
0 CO Rn 50 60 |
|
6 8 10 12 14 16 18 20 22 24 26 28 30 32 Air weight (kg/kg of fuel) |
 |
|
 |
|
|
2.6.1 Specific Volume of Flue Gases
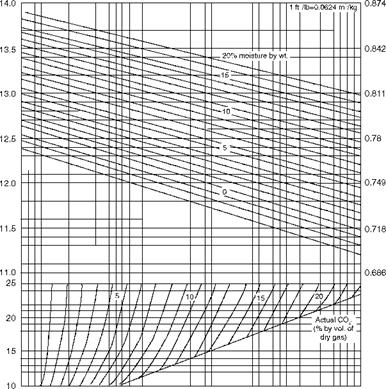
Figure 2.42 is very useful in calculating the specific volume of flue gas from the theoretical and actual CO2 (by volume on dry basis) at various moisture levels (by weight) in flue gas.
|
FIGURE 2.42 Specific volume of flue gas at NTP conditions. |



 19 августа, 2013
19 августа, 2013  admin
admin 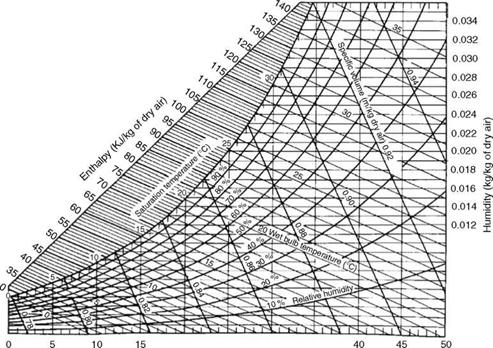
 Опубликовано в рубрике
Опубликовано в рубрике