Crude petroleum and its liquid residues left over in the process of distillation or cracking are the fuel oils used for steam generation. Crude petroleum is burnt for steam generation only infrequently, as the lighter fractions depress the flash point and present a fire hazard. Once the lighter fractions are removed, the oil becomes safe for handling and combustion. The petroleum-based liquid fuels are fuel oils, diesel oils, gas oils, kerosene, and gasoline or petrol.
With the increasing popularity of CCs, fuel oil-based power generation has gone out of fashion based on efficiency and few large oil-based utility boilers have been built except in refineries for consuming the heavy bottoms.
This term covers a wide range of oils starting from crude to the lightest products. These are now standardized into six ranges as shown in Tables 3.20 and 3.21.
1. Specific gravity is the ratio between the weights of oil and water at 15°C for the same volume. Usually it is expressed as specific gravity 15/15°C. API scale is more popular with oil industry and the relationship is as follows:
141 5
|
(3.3) |
![]() Specific gravity 15/15° C =——————
Specific gravity 15/15° C =——————
F B y ‘ 131.5+°API
|
Grade of Fuel |
No. 1 |
No. 2 |
No. 4 |
No. 5 Light |
No. 5 Heavy |
No. 6 |
|
Popular designation Usage Flash point |
Light: Domestic 38 |
Medium Domestic 38 |
55 |
55 |
55 |
Heavy Bunker C Industry 65 |
|
(min) °C Pour point |
0 |
-7 |
-7 |
_ |
_ |
_ |
|
(max) °C Water and |
Trace |
<0.1 |
<0.5 |
<1.0 |
<1.0 |
<2.0 |
|
Sediment volume (%) C residue on 10% |
<0.15 |
<0.35 |
||||
|
Bottoms (%) Ash (%) |
0.1 |
0.1 |
0.1 |
|||
|
By wt (max) Distillation temperatures 10% point C 90% point C Viscosity range SSU at 38°C |
<215 <288 |
282-338 |
45-125 |
150-300 |
350-750 |
900-9000 |
|
Seconds Saybolt Furol at 50°C API gravity (min) |
35 |
30 |
23-40 |
45-300 |
||
|
Sulfur (max) |
0.5 |
0.7 |
No limit |
No limit |
No limit |
No limit |
|
% 0.5 |
|
TABLE 3.20 |
|
ASTM Chart of Fuel Oil Properties |
![]()
|
Analyses of Fuel Oils |
![]() Note: Although the specific gravities of Grades 1 and 2 are specified, for Grades 4-6 they are not specified as they vary with the source of crude and extent of cracking and distillation.
Note: Although the specific gravities of Grades 1 and 2 are specified, for Grades 4-6 they are not specified as they vary with the source of crude and extent of cracking and distillation.
|
No. 1 |
No. 2 |
No. 4 |
No. 5 |
No. 6 |
|
|
Ultimate analysis (wt%) S |
0.01-0.5 |
0.05-1 |
0.2-2 |
0.5-3 |
0.7-3.5 |
|
H2 |
13.3-14.1 |
11.8-13.9 |
10.6-13 |
10.5-12 |
9.5-12 |
|
C |
85.9-6.7 |
86.1-88.2 |
86.5-89.2 |
86.5-89.2 |
86.5-90.2 |
|
N2 |
0-0.1 |
0-0.10 |
_ |
_ |
_ |
|
O2 |
_ |
_ |
_ |
_ |
_ |
|
Ash |
_ |
_ |
0-0.1 |
0-0.1 |
0.01-0.5 |
|
Other properties |
|||||
|
API gravity |
40-44 |
28-40 |
15-30 |
14-22 |
7-22 |
|
Pour point (°C) |
-18 to -45 |
-18 to -40 |
-23 to +10 |
-23 to +27 |
-9 to +30 |
|
Calorific value GCV (kcal/kg) |
10,930-11,030 |
10,650-10,970 |
10,155-10,777 |
10,555-10,565 |
9,670-10,550 |
|
TABLE 3.21 |
|
FIGURE 3.7 Variation of specific gravity of fuel oils with temperature. |
A. Specific gravity of 1 corresponds to 10°API. Variation of specific gravity with temperature is illustrated in Figure 3.7.
B. The higher the °API, the lower the density and lighter the oil.
C. Specific gravity has no technical significance except for conversion of weights to volumes. But this is important as the fuel oils are purchased by volume as barrels, gallons, or cubic meters.
D. Specific gravity determination is easy and instantaneous, as it involves merely dipping a hydrometer in the sample and reading the scale.
2. Calorific value can be expressed in megajoules per kilogram or kilocalories per kilogram and megajoules per cubic meter or kilocalories per cubic meter. The former is inversely proportional to the specific gravity, as the lighter oils contain more H. Gross calorific value varies from —10,200 to 10,800 kcal/kg. GCV measurement is done by bomb calorimeter. There are also following approximate relationships:
For uncracked distillate or residue GCV = 9810 + (38 X API gravity) kcal/kg For cracked distillate GCV = 9878 + (30 X API gravity)
3. Viscosity is a measure of resistance of fluids to flow due to the internal friction. In liquids this friction is due to the cohesion of molecules. As the cohesion decreases with increasing temperature, viscosity also decreases.
In the Saybolt Seconds Universal (SSU) test, viscosity is expressed as the time taken in seconds for the oil at a particular temperature to flow out of a 60 cm3 cup through a specified orifice. It is commonly measured at 100, 150, and 210°F (37.8, 65.6, and 98.9°C, respectively). For more viscous oils, Saybolt Furol viscosity is used in which the orifice is a little larger and the readings are roughly one-tenth of universal. Redwood seconds 1 and 2 (SR1 and SR2) are similar measurements in British practice.
Specific visocity is a comparison of times taken by the oil and water at 20°C to flow out of a 200 cm3 vessel and is expressed in degrees Engler. This is also a popular measurement in Europe.
|
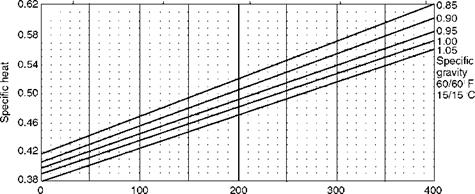
Temperature (°F) FIGURE 3.8 Variation of specific heat of fuel oils with temperature. |
However, none of the above-mentioned forms is a direct measurement of the true viscosity. Centipoise (N s/1000 m2) and centistokes (m2/106 s2) are the common measures of dynamic (or absolute) and kinematic viscosities.
Dynamic viscosity is the force that exists when a unit area of fluid is moving at unit velocity relative to that of a similar area, unit distance from and parallel to it. The dynamic viscosity of a fluid is the ratio of shearing stress to the rate of deformation. Kinematic viscosity is the dynamic viscosity divided by the density. Water at 20°C has dynamic and kinematic viscosities of 1 cP and 1 cSt.
Stokes = 0.00226 SSU — 1.95/SSU for 32 < SSU < 100 (3
= 0.00220 SSU — 1.35/SSU for SSU > 100
Figure 3.8 shows the variation of specific heat of various liquid fuels with
Temperature, whereas Figure 3.9 shows the manner in which the viscosity varies
With temperature. Equivalent viscosities are also given in Figure 3.9 for a ready reference. A similar figure appears in Chapter 10 (Figure 10.9) dealing with burner firing. There the figure contains parameters relevant to combustion.
4. Flash point is the lowest temperature at which sufficient vapor is produced to form an inflammable mixture with air under specified test conditions. Flash point is controlled solely for storage and gives no indication of burning properties of oil.
5. Pour point is the temperature at which oil starts to flow under test conditions, although it does not necessarily indicate the temperature at which oil stops flowing. Oils are not allowed to cool below this temperature in tanks from where the supplies are drawn; and in fact, oil should be stored at ~3°C higher than the pour point.
6. Carbon residue is the amount of carbon left behind after volatilizing the oil under specified conditions. It is the residue left after a fuel is heated out of contact with air. It is a measure of coke-forming tendencies of diesel oils. It has no significance with other fuel oils. Conradson and Ramsbottom are the two methods employed.
|
250 302 |
|
14 I_ |
|
50 |
|
|
200 100 50 30 24 10 7.5 |
|
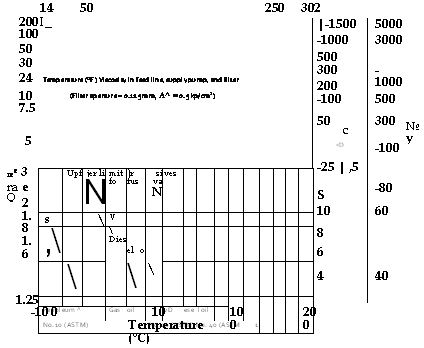
Temperature (°F) Viscosity in feed line, supply pump, and filter |
|
(Filter aperture = 0.125 mm, A^ = 0.5 kp/cm2) |
|
5 |
|
№ Y |
|
1.25 |
|
|
Ree 3 Ra e Q |
|
2 1.8 1.6 1.4 |
|
-10 0 |
|
10 Temperature (°C) |
|
100 |
|
200 |
|
O o 0) <J) T3 O O ■д ■a 0) Oc |
|
100 80 60 40- |
 |
|
|
|
 |
|
![]()
![]()
![]()
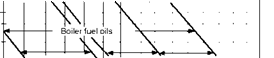
FIGURE 3.9
Viscosity versus temperature chart for various liquid fuels.
7. Sulfur affects low-temperature corrosion and chimney height. With high hydrogen content in fuel oils and consequent high water vapor in flue gases on combustion, there is —4% conversion of S into SO3, making the gases more acidic than the flue gases from coal firing with the same percentage of S.
Classification of fuel oils into six grades as per ASTM D396 is widely followed. Tables 3.20 and 3.21 summarize the classification.
• Grades 1 and 2 are called light and medium domestic fuel oils specified mainly by the distillation temperature.
• Grade 6 is called the heavy industrial fuel oil or bunker C oil and specified mainly by viscosity.
Naphtha is a general term used for spirits having low boiling points ranging from 30 to 170°C. It is used as a feed stock in fertilizer and petrochemical industries. Naphtha is a highly volatile product. It is made from
• Crude oil by direct atmospheric distillation
• Heavy residues by catalytic cracking
Naphtha essentially consists of paraffenic, naphthenic, and aromatic HCs. Naphtha is made in two varieties
1. HAN—high aromatic naphtha
2. LAN—low aromatic naphtha, also called petrochem naphtha
Naphtha is used to a limited extent as fuel in boilers and GTs. It is free of heavy and fouling elements such as vanadium, sodium, and lead. Sulfur is limited to 0.15%. Its GCV is >10,500 kcal/kg. Its volatile nature can be understood by its flash point of <23°C.
• Dry gas. Dry NG comes from wells away from oil deposits and it is free of heavy HCs.
• Sour gas. Natural gas containing some mercaptans and high amounts of H2S is known as sour gas.
• Sweet gas. Natural gas free of any of the above-mentioned undesirables is known as sweet gas.
From a combustion view, it is to be noted that the terms wet and dry NG have nothing to do with moisture in fuel but refer to the presence or absence of gasoline vapor. Natural gas is largely a mixture of HCs. Unlike coal or oil, NG has a higher H-C ratio and emits a lower CO2, which is beneficial in terms of global warming. The important properties are as follows:
1. Temperature. The temperature of NG, as it comes out of the well, varies from 0 to 75°C (—32 to 165°F) depending on the depth of the well. A rough indication is 1°C for every 30 m of depth (—1°F for 50 ft), which is not always realized in practice. At the plant gate, when it has traveled over a long distance from the well in the pipelines, the temperature is likely to be in the range of 5-25°C (—40 to 80°F) depending on the ambient conditions of terrain that it has traversed. For combustion purposes, it is normal to adopt a standard temperature of 15°C (60°F).
2. Moisture. At the head of the well NG contains no moisture unless there has been previous contact with salt water. In the gas pipelines, steam is added to rehydrate, that is, saturate with water vapor, to reduce the maintenance costs of pipelines. However, as the pressure is reduced at the time of distribution, the relative humidity drops. It is, therefore, the industrial practice to consider NG in a dry condition and at 15°C (60°F).
3. Density. This consists of the weights of individual constituents. Extreme accuracy in calculating the density and the CVs is not warranted, as the values of constituents keep changing. Calculated density is no more accurate than the volumetric analysis on which it is based. An analysis that groups all HCs as CH4 and C2H6 may indicate a lower density than the actual.
4. Gross calorific value. It is customary to compute and indicate the GCV of NG in kilocalories per cubic meter or megajoules per cubic meter (or Btu/ft[2]) at STP conditions of 15°C and 760 mmHg (60°F and 30 in. Hg) and in dry state. If the gas is saturated with moisture, the CV is to be reduced by 1.74%. Usually the GCV of NG is 10.35 kW/m3 or 8900 kcal/m3 (1000 Btu/ft3) in the range of 8.7-10.5 kW/m3 or 7500-9500 kcal/m3 (—850 to 1050 Btu/ft3). A much wider range of GCV is also possible when an extreme range of constituents is considered. The HV can be calculated by summing up the heat values of the various gas components, but it will be lower than the actual value determined by the bomb calorimeter as the unsaturated HCs are grouped as C2H6. The density computed also works out to be lower for the same reason. But the GCV on weight basis in kilocalories per kilogram works out to be very nearly the same as the result from the bomb calorimeter.
5. Constituents of NG. The principal constituents are methane (CH4) and the heavier paraffins such as ethane (C2H6), propane (C3H8), and butane (C4H10). It also contains 5-20% N2, and some NGs contain appreciable quantities of CO2 and H2S. Traces of argon, helium, and hydrogen are also present. O2 may be present if the gas is drawn from wells under suction, and air leakage is present in the pipelines.
|
TABLE 3.22 Typical Compositions of Natural Gases
|
H2S is substantially removed at the head of the well to prevent corrosion of piping. The heavy HCs that are in liquid form are also removed. Further, for ease of pipeline transportation, water and oil are sprayed to moisten. For combustion, therefore, as-delivered composition is more relevant than the well-head analysis.
6. Odorization. Generally, NG as it comes out of the earth is colorless and odorless and burns with a blue flame. It is highly explosive when mixed with air in right proportions. For this reason, odorization must be done to detect the presence of gas by adding traces of some organic sulfur compounds.
Typical composition of NGs is given in Table 3.22.
• There is a wide variation in the composition, density, and CVs.
• Theoretical CO2 varies approximately from 12 to 15% at stoichiometric conditions.
• Stoichiometric air requirement can be substantially constant at 1.335 kg/ 1000 kcal of GCV despite large variation in composition.
Large NG reserves are found in Russia, Iran, Qatar, United Arab Emirates, Saudi Arabia, the United States, Algeria, Venezuela, Nigeria, and Iraq. There are small reserves in several parts of the world. Large importers of NG are mainly the United States, Germany, Ukraine, Italy, and Japan, whereas the exporters are Russia, Canada, Algeria, Norway, Middle East, and Indonesia.
Liquefied natural gas is a relatively new fuel gaining popularity since the mid-1960s. Unlike oil, which packs a lot of heat in a small volume, NG is bulky and relatively difficult to transport. This deficiency is largely removed in LNG.
Natural gas is liquefied at -162°C and transported either by pipelines or by large ships. NG needs liquefication and loading facilities at the production end and regasification and distribution facilities at the consuming end. A ton of LNG is equivalent to nearly 1500 m3 of NG of 8900 kcal/m3 of CV or 2.5 of coal with 5300 kcal/kg of CV.
Algeria, Libya, Middle East, Southeast Asia, and Alaska are the major exporters, with Algeria and Libya exporting to Europe and the United States and the others exporting to Japan, South Korea, and Taiwan.
3.4.2 Waste or Manufactured Fuels
Refinery Gas
Refinery gas (RG) is the by-product of both distillation and cracking processes in the refinery. Distillation is the process of separating various components of petroleum by their specific gravities when the oil is heated to -300°C and flashed in a fractionating column. Distillation can occur under vacuum or pressure and in the presence of steam or gas.
Cracking is the thermal decomposition of the oil heated at 450-550°C and 50 kg/cm2 pressure and discharged into separators and then to the fractionating tower.
Source. RG is withdrawn from any point in the system:
• From raw gasoline tanks, stabilizers, cracking stills, or adsorbers used for purification
• At below or above atmospheric pressure
• At room temperature or much higher, if taken from the cracking still or fractionating tower
Often RGs are blended and supplied, which is the reason for the wide variation in analyses.
Composition. RG is composed of mainly paraffins with low boiling point—CH4, C2Hg, C3H8, C4H10, and C5H12— and small amounts of olefins. Minor quantities of CO2, CO, and H2 may also be present along with O2 and N2 at times introduced to lower the pressure of HCs in the cracking process. Sulfur of the original crude appears as SO2 unless it has been removed. Typical analyses and properties of RG are given in Tables 3.23 and 3.24.
Coke Oven Gas
Coke oven gas (COG) is the waste produced in the destructive distillation of the bituminous coal in the manufacture of coke. Coke oven batteries are charged with coking coal and heated externally to drive out all the VM. Products such as ammonium sulfate, oils, and tars are recovered. The incondensable gases are collected and supplied as COG, which is -15% by weight of the coal charged into the slot-type coke ovens.
The composition of the gas is dependent on the analysis of raw coal and the length of the time of coking. Mainly the COG consists of H2, CH4, C2H4, and CO with small amounts of
|
TABLE 3.23 Refinery Gas Analysis by Percentage Volume at 15°C, 760 mm wg
|
|
Typical Coke Oven Gas Analysis on Cubic Meter Basis Range Typical
|

|
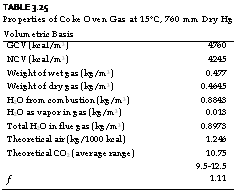
TABLE 3.25 Properties of Coke Oven Gas at 15°C, 760 mm Dry Hg Volumetric Basis
|
 CO2, N2, O2, and heavy HCs. COG comes out at high temperatures and contains suspended particles of tar, dust, benzol, and H2S. It is usually washed and cooled before sending to COG holders. A substantial portion of the COG is used for heating the coke ovens. More than half of the gas is used by the steel companies in boilers. There are two types of coke commonly made: (1) foundry coke and (2) blast furnace coke.
CO2, N2, O2, and heavy HCs. COG comes out at high temperatures and contains suspended particles of tar, dust, benzol, and H2S. It is usually washed and cooled before sending to COG holders. A substantial portion of the COG is used for heating the coke ovens. More than half of the gas is used by the steel companies in boilers. There are two types of coke commonly made: (1) foundry coke and (2) blast furnace coke.
• High-temperature carbonization at ~980°C is adopted for making foundry coke and at 1400°C for making blast furnace coke. Typically a ton of coal with —31% VM would yield 720 kg of coke and 330 m3 of gas, besides tar, water, light oils, and NH3. The GCV value lies between 4.13 and 6.22 kW/m3 or 3550 and 5350 kcal/m3 (—400 to 600 Btu/ft3).
• Low-temperature carbonization yields lesser amounts of gas but with higher CV of 5350-8900 kcal/kg (600-1000 Btu/ft3).
Coke oven gas burns readily because of free H2 and presents no problems in combustion. But the other impurities in gas deposit themselves in the pipelines and burner openings. The burners are required to have larger gas-port openings and easier access for cleaning. Typical analysis and properties of COG are given in Tables 3.25 and 3.26.
Blast furnace gas (BFG) is the waste product from the conversion of iron ore into pig iron. A blast furnace is charged with ore, coke, and limestone from the top sequentially, and hot air at 600-700°C is blown from the bottom openings. Air travels upward, and while the charge descends, creating an intimate contact, coke reacts with O2 in air to form CO with some heat. CO reduces the iron ore at this high temperature and frees carbon to form iron and CO2. Limestone reacts with the products to remove the impurities and in this process it forms more CO2.
The BFG that exits at the top of the blast furnace contains CO and CO2 in good measure, along with N2 and H2 and traces of CH4. N2 is from air whereas H2 and CH4 are from the dissociation of water vapor at high temperature inside the blast furnace. The gas is hot (150-425°C), dusty (iron oxide), and low in CV.
The gas pressure varies between 500 and 750 mm w. g. Unfortunately, this pressure varies widely due to the violent movements inside the blast furnace and switching of blast stoves.
|
TABLE 3.27 Lethal Nature of Blast Furnace Gas
|
This variation is passed downstream and a good solution would be to install a gas holder. But gas holders are both costly and space-consuming.
The dust at the top can be as high as 115 g/dry sm3 (dsm3) [50 grains/dry scf (gr/dscf)] comparable to CFBC or PF upsteam of filter. Since this dust is high, the gas is passed through a mechanical dust collector to reduce loading to 7-35 g/dsm3 (3-15 gr/dscf) followed by one more dry or wet cleaning, which is performed to reduce the dust to <700-2300 mg/dscm (—0.3 to 1.0 gr/dscf). The third stage involves a more vigorous washing or electrostatic precipitation when the dust loading is down to 10-20 mg/dscm (0.005-0.01 gr/dscf).
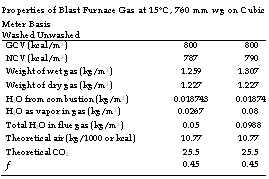
Blast furnace gas is a heavy gas (about 1.25 times heavier than air) with low GCV, due to large amounts of N2 and CO. There is practically no sulfur and water vapor on combustion, eliminating any threat of corrosion, both at high and at low temperatures. Older plants yield richer gas, whereas the newer and more efficient plants produce leaner gas. Gas pressure, quantity, and quality vary a great deal, necessitating a support fuel all the time. But the stoichiometric air requirement at —10.8 kg/1000 kcal/kg of GCV is remarkably constant.
Blast furnace gas requires a high temperature of —800°C for ignition and burns at —1100 to 1200°C in a boiler with unheated air. The flame propagation velocity is low, between 25 and 32 m/s at stoichiometric conditions. The gas produces a faintly bluish and almost nonluminous flame with pinkish tinge at the end. This gives a confusing picture of the flame-out condition, making the situation very dangerous, as the leakage of BFG is lethal due to its high CO content. This is captured in Table 3.27. Flame stability is to be ensured by radiation from refractory. Cofiring with other fuels is the easiest way to mitigate the safety issues.
Both dusty dry and wet clean gases are used in boilers with different problems.
• Dusty gases choke the gas passes and burners, also causing erosion and fouling of the HSs.
• Wet gas deposits are too hard to remove.
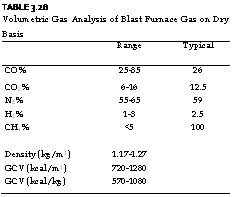
In a way dustiness is a better nuisance than wetness. Typical analysis and properties of BFG are given in Tables 3.28 and 3.29.
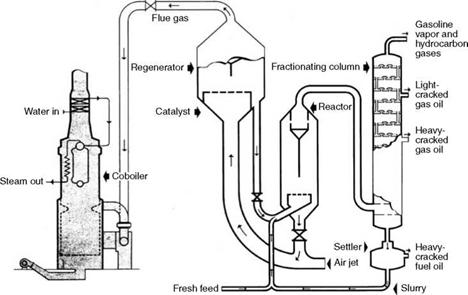
CO Gas
During the catalytic cracking of petroleum with a thermofor catalytic cracker (TCC) or fluid catalytic cracker (FCC), the catalysts must be scrubbed clean to remove the coke deposited by the cracking process. This regeneration of the catalyst is performed by burning away the carbon in the regeneration tower with the help of large quantities of high-pressure air at —2 kg/cm2. This air has to be kept to a minimum, in fact to substoichiometric levels, to
|
FIGURE 3.10 Schematic of CO gas formation. (From Doosan Babcock Energy Ltd., U. K. With permission.) |
|
Properties of Blast Furnace Gas at 15°C, 760 mm wg on Cubic Meter Basis Washed Unwashed
|
|
TABLE 3.28 Volumetric Gas Analysis of Blast Furnace Gas on Dry Basis
|
 Contain the temperature to prevent the destruction of catalyst and minimize the compressor power. The combustion therefore produces a good amount of CO and hence this waste gas is termed CO gas.
Contain the temperature to prevent the destruction of catalyst and minimize the compressor power. The combustion therefore produces a good amount of CO and hence this waste gas is termed CO gas.
CO gas leaves the regenerator at a temperature as high as 600-700°C and naturally contains a large amount of sensible heat, although the GCV is <350 kcal/m3 with CO content of 4-10% by volume. CO-CO2 oxidation takes place in a CO boiler. Catalytic dust carried over is in traces, <40 jam in size. The ignition temperature of CO is 610-660°C and stable combustion takes place if the adiabatic flame temperature is 980°C. An auxiliary fuel with 10% capacity generally fills this need.
CO gas is consumed in the refinery by producing the process steam in the CO boilers that incidentally attenuate the noise and discharge CO2 instead of toxic CO (Figure 3.10). Typical analysis and properties of CO gas are given in Table 3.30.
|
TABLE 3.30 Properties of CO Gas on Volumetric Basis
|
Ash Analysis
Constituents of ash display the following features. They
• Are almost entirely metal oxides
• Vary widely
Ash analysis can be understood in either (1) chemical or (2) mineralogical terms, or preferably both, to gain a complete picture. Ash is actually a mixture of various mineralogi — cal compounds, whose analysis is not easily amenable to standard laboratory methods as chemical analysis. Although the chemical analysis captures all the oxides that make up the ash, it does not tell us in what form they actually exist in the ash.
The chemical constituents of ash are metal oxides. ASTM D3124 provides guidelines for testing a sample of powdered coal required to be burnt in a ceramic crucible to completion at 700-750°C. Chemical analysis is done to determine each constituent and report weight percent. The ash composition varies so widely that there is no standard analysis to benchmark. Coal ash analysis is different from the analysis obtained for bottom ash, fly ash, or furnace slag. Enough data is now available to predict the behavior of ash in a boiler in a reasonably accurate manner. Table 3.31 gives a range of analysis for various constituents of ash.
Understanding the behavior of ash based on the original MM forms is the purpose behind mineralogical analysis. The testing procedure is different. More than 100 mineral species are associated with coal and the reactions among them are complex. Mineralogi — cal analysis can provide clues to certain aspects of ash behavior not fully understood with the aid of chemical analysis. Calcite (CaCO3), dolomite (CaCO3 ■ MgCO3), siderite (FeCO3), pyrite (FeS2), gypsum (CaSO4 ■ 2H2O), quartz (SiO2), hematite (Fe2O3), magnetite (Fe3O4), and rutile (TiO2) are some of the minerals.
• Ash high in acidic constituents such as SiO2, Al2O3, and TiO2 has high softening temperature, not affected much by reducing temperature.
• Basic oxides such as Fe2O3, Na2O, K2O, CaO, and MgO tend to reduce the softening temperature.
• Iron compounds are problematic as S in FeS2, in its passage through the furnace, dissociates and reacts with O2 to form SO2 and SO3.
• S also combines with Na and K to form low-melting compounds that cause corrosion and slagging.
|
TABLE 3.31 Range of Chemical Constituents of Ash
|
Coal Ash Fusibility
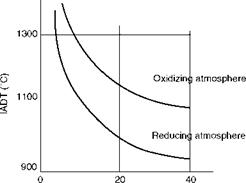
Ash fusibility lends itself to an empirical method of predicting the ash behavior at high temperature so that the sizing of a furnace is done correctly. ASTM D1857 gives the procedure in which the gradual deformation of an ash pyramid, 19 mm high with 6.35 mm equilateral base mounted on a refractory base, is heated in a gas or electric furnace, in reducing and oxidizing atmospheres, at a specified rate to record the four characteristic deformation temperatures shown in Figure 3.11.
1. Initial ash deformation temperature (IADT) is the temperature at which the specimen cone begins to soften at the top.
2. Softening temperature (ST) is the temperature at which the cone shape is lost and the height and breadth become equal.
3. Hemispherical temperature (HT) is the temperature at which the cone reduces to a hemispherical shape.
4. Fluid temperature (FT) is the temperature at which the specimen is flat and the molten ash begins to flow.
Initial ash deformation temperature is the most important for boiler design and operation. Softening temperature is often noted, as the stickiness of ash starts increasing at this point.
• Normally the difference between IADT and ST can be 100°C.
• The permissible difference between two runs may be as high as 55-85°C. Standards followed by other countries also differ and great care is needed in using the data.
• All ash deformation temperatures are lower in reducing conditions, and iron compounds have a great influence in depressing the temperatures. Figure 3.12 illustrates the point in a qualitative manner.
• Furnace exit temperatures should be designed for at least 55°C lower than the IADT in reducing conditions.
Ash Viscosity
The viscosity of molten coal ash can be measured in a rotating-bob viscometer, but it requires a lot of ash and the procedure is very time-consuming and expensive. Fortunately, it can be estimated from the chemical analysis of the ash with sufficient reliability. Ash up to viscosity of 250 P can be removed from the operating boilers. The temperature to attain such level is referred to as T250, which is a benchmark. The following ratios are used to estimate the viscosity, depending on the type of ash.
Q-f-v
Silica percentage (SP) =—————————- i—2 X 100 (3.5)
|
FIGURE 3.11 Four stages of ash deformation temperatures. |
![]() SiO2 + Equivalent Fe2O3 + CaO + MgO
SiO2 + Equivalent Fe2O3 + CaO + MgO
|
|
|
Total Fe in ash (%) FIGURE 3.12 Effects of iron compounds and reducing conditions on IADT. (From Babcock & Wilcox Co., U. S.A. With permission.) |
Where
SHAPE \* MERGEFORMAT ![]()
|
(3.6) (3.7) (3.8) |
![]() Equivalent Fe2O3 = Fe2O3 + 1.11FeO + 1.43Fe
Equivalent Fe2O3 = Fe2O3 + 1.11FeO + 1.43Fe
Fe2O3 + CaO + MgO + Na2O + K2O
Base-acid ratio (B/A)
SiO2 + AI2O3 + TiO2
|
Dolomite% |
![]() CaO + MgO
CaO + MgO
X 100
CaO + MgO + Na2O + K2O + Fe2O3 B/A ratio in the range of 0.4-0.7 denotes a high slagging potential.
The ill effects of ash on boiler design have already been mentioned:
1. Erosion
2. Slagging and fouling
3. Corrosion
Erosion of Boiler Parts due to Fuel Ash
Erosion is a form of wear. Wear can be defined as progressive loss of a surface material due to mechanical action involving impingement of abrasive particles. Unlike corrosion, which is a chemical or electrochemical action, wear is purely mechanical. Readers may refer to Chapter 5 on wear-resistant materials. Abrasion and erosion are two types of wear.
1. Abrasion can be likened to sand-papering in which solid particles move in contact with a parallel surface. Abrasion affects the high spots of the surface without much effect on the main body. The resulting loss of material is smaller in comparison with erosion. Abrasion resistance can be built by a boundary layer of high and preferably hard spots.
2. Erosion is the impingement of hard particles at an inclination, and it has more energy and destructive power than abrasion. The impinging particles cut through the boundary layer and destroy the main matrix. Hence, abrasion-resistant material cannot withstand erosion.
Wear is not a problem that afflicts the oil — and gas-fired boilers. It is an issue only with solid fuel firing, as wear occurs on both fuel and ash sides of the boiler and handling plants. The pulverizers and the coal piping of PF-fired boilers experience wear based on the ash content and constituents. High-ash coals tend to cause wear and the normal solution is to
• Make the wear parts from wear-resistant materials
• Provide sacrificial materials at appropriate places
These methods are described in Chapter 13 dealing with PF boilers. Wear is also experienced in coal bunkers and feeders, and usage of wear liners is a proven solution.
Erosion of hot parts due to ash, particularly the tubes, is a serious problem affecting the availability of solid fuel-fired boilers. The hard constituents of ash, namely, Al2O3 and SiO2, moving with flue gas at high velocities impinge on tubes, refractory, and other parts in the gas path, causing erosion. Ash, as originally present in coal, is not so abrasive. But after it melts due to high furnace temperatures and recrystallizes, the abrasiveness increases dramatically and the ash is re-formed into crystals with their characteristic sharp edges. FBC ash is less erosive, as it does not melt and hence does not reshape into hard crystals, at least not to the same level of PF. But the amount of ash in bed in BFBC and in recirculation in CFBC is so high that it can cause significant erosion damage to both tubes and refractory. Bed-coil erosion in BFBC and erosion of furnace walls, tube banks in the first pass, and cyclone refractory in CFBC are extremely severe. Erosion is influenced by the following:
• Flue gas velocity.
• Flue gas dust loading.
• Nonuniformity of dust loading and gas velocity across the cross section.
• Density of tubes in a bank.
• Tube disposition in a bank—inline or staggered.
• Gas turns that segregate the ash due to centrifugal action.
• Angle of ash particle impingement. Normal and parallel to the surface, the erosion is found to be minimum, whereas between 20 and 30°C in horizontal to the surface, the wear is found to be maximum.
Erosion is an inseparable aspect of boilers firing solid fuels. It can be minimized but not eliminated altogether. The aim is to minimize and predict the erosion rate so that the intervals between the downtimes can be extended to coincide with the planned outages, thus improving the unit availability. The erosion prevention and protection measures essentially fall into two categories:
1. Design stage measures
2. Provision of sacrificial protective material
Erosion prevention is essentially achieved at the design stage, mainly by adopting measures that reduce the severity of contact between ash and PPs. Erosion protection is achieved by providing sacrificial protective material. Further, erosion in the boiler is
Monitored during operation and sacrificial material is increased if erosion persists. This is an on-site protective measure.
1. The design stage erosion prevention measures are as follows:
A. Gas velocities. Limiting the flue gas velocities is the most important design step to eliminate external tube or refractory erosion. Guidelines for maximum permissible gas velocities are given in Chapter 6.
B. Inline tubes. Tube disposition is another major point to be noted. Inline tubes reduce erosion dramatically as compared with a staggered arrangement.
C. Bigger furnace. Limiting the dust loading is done by sizing the furnace chamber generously and providing adequate SA and TA with a strategic placement of nozzles.
D. Tower-type boiler. Avoiding the gas turns is another important design step. The centrifugal force segregates and hurls the ash toward the edges, causing damage to the PPs in the path. It is preferable, for example, to avoid two-pass boiler banks (BBs) for abrasive fuels. Tower-type boilers are preferred by many customers, as they offer protection against such damage.
2. The sacrificial erosion protective measure is a wear-resistant liner or stud material either attached or welded to the affected tubes. This can be an established design measure or a new preventive measure. In both cases, the erosion patterns have to be carefully studied and appropriate patterns have to be evolved. Possible side effects should also be neutralized.
A. Wear liners are likely to render certain amount of tube surface ineffective, whereas studding is likely to increase the effectiveness; both measures call for adjustments of the HS.
B. Liners and studs protect the surfaces on which they are placed but they are likely to deflect the ash streams elsewhere, thereby shifting the problem instead of solving it.
C. Studs are attached to the tubes by welding and hence they are kept cool. They are usually made of carbon steel (CS). When long studs are needed the cooling efficiency is reduced. The studs should be made of alloy or stainless steel (ss).
D. The liners are of CS when they are used for protecting low-temperature sections such as the economizer (ECON) in the second pass of the boiler. But at high temperatures as required in SH, they are made from ss and, at times, from high-temperature alloys like Si-Cr-Al steels. The liners are not welded to the tubes to avoid damage due to differential expansion between the tube and the liner. Since there is no cooling, the liners attain nearly the same temperature as the gas and expand much more than the tube. Unless attached to the tube at close intervals the liner sags and fails to provide proper protection.
Although it appears to be simple to attach a liner to the tube, it is a difficult task in practice. A comprehensive list of proven erosion protection measures is given in Figures 3.13a and b. They should be adopted with great caution.
|
Designation |
Principle |
Remarks |
||
|
Protective Strips |
Liner |
Pure deformation wear for high ash in flue gases |
||
|
Protective Shells |
Ј8 |
Anchor Curved Liner |
For deformation and cutting wear with slightly lower ash in flue gases |
|
|
Studding and Refractory Lining |
MU |
For tube walls and inbed tubes of BFBC with no refractory |
||
|
Square tubes |
1 + p — ♦ |
For very high ash like in CFBC suited for heavy abrasive wear with parallel flow |
||
|
Omega tubes |
5 |
|||
|
Protection of tube bends |
♦ Ill 4~u4 |
Wear »’^hood |
Suited for protection of tube banks after gas turns |
|
|
(a) |
![]()
|
Remarks |
![]()
|
Principle |
![]() Designation
Designation
|
Slotted Plate |
![]()
|
Gap cover |
![]() This arrangement minimizes high velocity in the lanes
This arrangement minimizes high velocity in the lanes
|
(A) 4h|hM>^ < H H M M ► I Better |
|
(B) MкM |
![]()
![]()
Tube arrangement in-line versus staggered
Erosion of second and following tubes minimized with in-line arrangement
|
|
|
|
|
|
|
|
![]()
|
|
||
|
|||
|
|||
|
(b) |
![]() Better Worse
Better Worse
FIGURE 3.13
(a) Special tubes and liners for wear resistance. (b) Tube arrangements for better wear life.
Slagging and Fouling
Both processes produce high-temperature ash deposits.
• Slagging is the formation of fused slag deposits on furnace walls and other surfaces exposed to radiant heat.
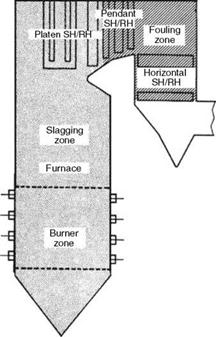
• Fouling is the formation of bonded (sintered or cemented) deposits in mainly SH and reheater (RH) areas from convection heat (Figure 3.14).
Depending on the fusibility, the various compounds of ash can be classified into three categories:
1. High-melting compounds such as SiO2, Al2O3, Fe2O3, CaO, and MgO, which are
A. Pure oxides with fusibility ranges of 1600-2800°C that do not melt but end up in fly ash and retain their original structure
B. A cause for erosion but not slagging
2. Medium-melting compounds such as Na2SiO3, K2SO4, and FeS, which
A. Have a fusibility range of 900-1100°C
B. Form the base sticky layer on water walls and platens
C. Cause slagging
3. Low-melting compounds, mainly chlorides and sulfates of alkali metals, such as NaCl, Na2SO4, CaCl2, and MgCl2, which
A. Have a fusibility range of 700-850°C
B. Form the base sticky layer on SH and RH tubes
C.
|
FIGURE 3.14 Slagging and fouling zones in a PF boiler. |
![]() Cause fouling
Cause fouling
|
|
• In slagging that takes place on furnace, division walls, and platens, the molten ash particles moving in the flue gas stream solidify on contact with tube metal, which is cooler, and form a loose flowing layer. Short retractable SBs or wall deslaggers remove these from a furnace. Long retractable blowers are needed for platens. At times the inner layer attaches itself to the tubes firmly and the outer layer grows in size till it detaches by its own weight and falls on the furnace bottom, causing serious damage such as destroying the floor. Viscosity of ash determines the ease of deslagging.
• In fouling that takes place in the tube nests, the action is different. The base sticky layer is formed by the solidification of ash on cooler tubes. Here, the ash layer is not flowing. The temperatures of the outer layer of ash are higher but still cool enough for further ash to solidify and attach itself by fusing. After some growth the ash particles do not solidify, as the temperatures are too high and an equilibrium depth of deposit is reached. These ash deposits are removable by soot blowing. At times, with certain coals, the sintering of ash is very strong, making it difficult to dislodge the deposits with soot blowing.
Slagging and fouling are interlinked. When the furnace slags, the ash deposits reduce the heat transfer and raise the gas temperatures which, in turn, cause more fouling.
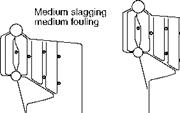
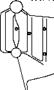
Slagging and fouling characteristics have profound influence on sizing the furnace and SH/RH. When a particular coal is highly fouling, the furnace volume should be increased to get the gas temperatures low enough not to cause slagging. Figure 3.15 illustrates the effect. Another solution is to have gas tempering (GT) in which low — temperature flue gas from ECON exit is introduced below the furnace outlet. These measures are expensive.
|
|||
|
|||
|
|
||
|
|||
|
|||
|
|||
|
|
||
![]()
![]()
![]()
![]()
![]()
![]()
|
|
![]()
![]()
FIGURE 3.15
Effect of slagging and fouling on furnace size and soot blower location.
|
|||
|
|
||
|
|||
|
|||
Slagging and fouling are controlled by the following:
1. Furnace dimensions
2. SB locations
3. Spacing and bank depth selection of the SH and RH banks.
Besides the ash fusibility and viscosity the following ratios also affect slagging and fouling properties.
• Base-acid ratio between 0.4 and 0.7 is a low fusibility range and ash has a higher slagging potential.
• Silica-Alumina ratio ranges from 0.8 to 4.0. At >2.8 the fluid temperatures decrease.
• Iron-calcium ratio is the ratio of Fe2O3/CaO. Among the five basic oxide compounds Fe and Ca are the most important and abundant. Fe2O3 constitutes 5-40% and CaO constitutes 2-30%. Between 10 and 0.2% the ash fusibility is markedly lower.
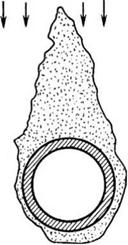
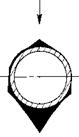
Loose deposits in the low-temperature zones, like first-stage SH or ECON with gas temperatures <600°C, ash in flue gas is in solidified form and does not stick to the tubes but forms a friable deposit, which is easily blown away by the action of SBs.
Ash deposits build on the front side of the tube in case of slagging and fouling, whereas it forms on reverse side for the friable deposits. Also, these deposits are velocity governed— less on higher velocities and more on lower velocities. Hence, very low gas velocities of ~5 m/s are not recommended (Figures 3.16 and 3.17).
Corrosion is the accelerated destruction of the metal by chemical or electrochemical action of salts. While a more detailed and general description of various types of corrosion in
Approximate Limiting Tube Metal Temperatures for Various Gas Temperatures
Gas temperature (°C) 1300 1250 1200 1150 1100 1050 1000 950 900
Limiting metal 560 565 570 575 585 595 610 630 650
Temperature (°C)
Boilers is presented in Chapter 5, only the external corrosion of tubes due to ash is elaborated in this section.
Ash deposits that form on the high — and low-temperature areas of the boiler are responsible for slagging and fouling and corrosion, although the deposits need not necessarily lead to these problems. Corrosion due to coal ash takes place because of the attack of the molten ash salts in the innermost layer of the ash deposit next to the tube. It is rarely found in SH and RH tubes that contain only dusty deposits. The places of corrosion attack are the following:
• Economizer and airheater (AH), arising from low-temperature corrosion
• Furnace SH and RH areas, caused by high-temperature corrosion
Low-Temperature Corrosion
Causes for low-temperature corrosion are well understood. Sulfur from coal volatilizes at high temperature and combines with oxygen in air to form SO2 and SO3 that pass through the boiler and at low temperatures of <200°C react with the water vapor to form sulfurous and sulfuric acids that cause corrosion. This is elaborated further in Chapter 6.
High-Temperature Corrosion
In contrast, high-temperature corrosion of coal ash is not fully understood. The reasons and mechanisms of corrosion are subject to different interpretations. However, there is consensus on the preventive measures to be adopted to avoid the damage. Corrosion is measured by the reduction in tube wall thickness in microns per year.
• Coals with sulfur >3.5% and chlorine >0.25% attack SH and RH tubes.
• High tube metal temperatures were thought to cause corrosion, but trouble-free working with several high-temperature SHs has brought about an understanding that the combination of both high gas and metal temperatures causes corrosion. Table 3.32 gives approximate limiting safe metal temperatures.
• External corrosion of SH and RH takes place on the upstream side of the tube.
• Corrosion rates are lower in case of austenitic steels such as 18 Cr-8 Ni as compared to ferritic alloy steels (AS) such as 2.25% Cr-1% Mo.
• Corrosion rates are nonlinear, rising sharply above 620°C metal temperatures and peaking between 680 and 730°C before falling sharply.
• Outlet tubes of radiant SH and RH platens, unlike retractable SBs, experience the highest corrosion rates.
The distillates are free of ash; the residuals concentrate all the ash (with its metallic compounds) and large part of the sulfur of the crude oil. Even then the ash scarcely exceeds
0. 2% by weight. This amount of ash in comparison to coal ash may not be impressive but the problems caused are significant.
• Slagging and corrosion of SH and RH
• Low-temperature corrosion of ECON and AH
Na and V are the most important metallic constituents of ash, as they form complex low — melting compounds and vary from traces to 30% each. Sulfur, the other problematic element, varies from 0.7 to 5%. The melting temperatures are between 250 and 680°C (which coincide with the tube metal temperature range) with no sharp melting point but softening and melting over a wide temperature range. The ash constituents are the same as in coal ash with the addition of V and Ni oxides.
Predicting the slagging and fouling tendencies from ash analysis is largely a matter of experience based on the following:
• Ash quantity
• Na and V levels in ash along with other major constituents and their melting temperatures
• S in ash
With tube metal temperatures restricted to 540°C, progressive fouling of furnace and SH does not occur if the combustion conditions and soot blowing practices are in order.
Sulfur is responsible for corrosion at both
1. High temperature—affecting SH and RH tubes due to low-melting ash deposits, which are corrosive when melted
2. Low temperature—affecting ECON, AH, flues, and dust collector due to sulfuric acid from flue gases
It is important to note that high-temperature corrosion rarely occurs at metal temperatures <600°C. SH operating at 540°C is in danger, as the metal temperatures are in the vicinity of 600°C, and several precautions should include
• Oil treatment to remove V, Na, and S
• Use of additives
• Use of corrosion-resistant alloys and coatings
• Low excess-air operation
Maintaining the metal temperature above the acid dew point, the temperature at which the flue gases are saturated with H2SO4, prevents low-temperature corrosion. Reducing the formation of SO3 by lowering the excess air and treatment of oil with additives are more positive approaches.
Further Readings
Attig, R. C. and Duz, A. F., 1979, Coal ash deposition studies and application to boiler design, American Power Conference, Chicago.
Black, K. P. and Veatch, R. M., 1996, Power Plant Engineering, Chapman & Hall, New York.
Blazewicz, A. J. and Gold, M., 1979, High Temperature Gas Side Corrosion in Coal Fired Boilers, ASME, December.
Braunkohle International-Die wichstigsten Forderstaaten im Uberblick, Rheinbraun informiert.
Burbach, H. E. et al., 1977, Compatibility between furnaces and fuels conducive to high boiler availability, Power, Combustion Engineering Inc., December.
Graham, J. D., 1984, Biomass fuels for steam generation, B&W Canada, American Power Conference, April.
Lisowski, J., 1978, Practical Aspects of Burning Blast Furnace Gas in Boilers, Peabody Holmes Limited, Power and Works Engineering, September.
Power from oil, Special Reports from Power, October 75 to October 76.
Singer, J. G. and Blackburn, S. S. Tangential Firing of Regenerator Waste Gas in CO Boilers, Combustion Engineering, USA, ASME Paper 60-WA-327.
Singer, J. G. Combustion Fossil Power, 4th Edition.
Smith, A. D., 1976, Wood as a Fuel, Foster Wheeler Ltd., Canada, May.
Smith, V. L., 1977, Catalytic regenerator gas discharge (CO)—valuable as fuel, B&W, Utility Seminar, Sao Paulo, Brazil, November.
Steam, Its Generation and Use, Various Editions.
Swanekamp, R., 1996, Fuel management: natural gas/fuel oil, Power, Special Report, January.
The Efficient Use of Fuel, 1958, HMSO, U. K.
Vecci, S. J. and Olson, G. B., 1978, Fuel and ash characterisation and its effect on the design of industrial boilers, American Power Conference, April.



 20 августа, 2013
20 августа, 2013  admin
admin 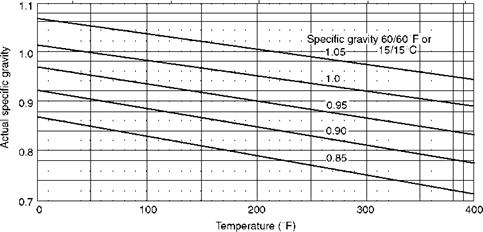

 Опубликовано в рубрике
Опубликовано в рубрике