Hydrogen and ammonia are valuable chemicals in various processes. The steam reforming process is widely used to produce hydrogen from fossil fuels such as natural gas, oil, or even coal as shown in Fig. 2.10. There are several variations of the process, but basically the steam reforming process converts a mixture of hydrocarbons and steam into hydrogen, methane, and carbon dioxide in the presence of nickel catalyst inside tubes. Before entering the reformer, the natural gas has to be desulfurized in order to protect the reformer tubes and catalysts from sulfur poisoning. The desulfurized gas is mixed with process steam, preheated to about 500°C in the flue gas boiler, then sent through the tubes of the reformer. Reactions occur inside the tubes of the reformer at 800-950° C.
|
Figure 2.10 Steam reforming process in hydrogen plants. 1, natural gas; 2, sulfur removal; 3, reformer; 4, reformed gas boiler; 5, flue gas boiler; 6, shift converter; 7, air preheater; 8, air; 9, CO2 removal and methanation; 10, Pressure Swing Adsorption (PSA); 11, H2 product; 12, stack; 13, CO2 by-product. |
Reforming pressures range from 20 to 40atm, depending on the process equipment supplier.
CnHm + nH2O! nCO + (m/2 + n)H2
CH4 + H2O ^ CO + 3H2
CO + H2O ^ CO2 + H2
The overall reaction is highly endothermic, so the reaction heat has to be provided from outside by firing fuel such as natural gas or naphtha outside the tubes. This generates flue gases, typically at 1800°F and atmospheric pressure, that are used to generate high pressure superheated steam in a water tube waste heat boiler, generally referred to as a flue gas boiler. The flue gases also preheat the steam — fuel mixture and air.
In some processes the effluents of the primary reformer are led to the secondary reformer, where they are mixed with preheated air. Chemical reactions occur, and the catalysts convert the methane partly to hydrogen. The effluent from the reformer, called reformed gas, is at a high gas pressure, typically 20-40 atm, and contains hydrogen, water vapor, methane, carbon dioxide, and carbon monoxide. This gas stream is then cooled from about 1600°F to 600°F in a reformed gas boiler, which is generally an elevated drum fire tube boiler (Fig. 2.11) with provision for gas bypass control to maintain the exit gas temperature constant at all loads. The exit gas temperature from the boiler decreases as the duty of the boiler decreases, and the bypass valve adjusts the flow between the incoming hot gases and the cool exit gases to maintain a constant exit gas temperature at all loads. The cooled gases then enter a shift converter, where CO is converted to CO2 in the presence of catalyst and steam. Additional hydrogen is also produced. The exothermic reaction raises the gas temperature to about 800°F. The CO content is reduced from about 13% to 3%. Awaste heat boiler referred to as a converted gas boiler cools the gas stream before it enters the next stage of conversion, where CO is reduced to less than 0.3%. The next stage is the methanator, in which catalysts convert traces of CO and CO2 to methane and water vapor. The H2, CO, and unreacted methane are then separated. This produces a gas stream that can be recycled to process feed and produce hydrogen of 98-99% purity that is further purified by the pressure swing adsorption method. In older plants carbon dioxide is removed in a liquid absorption system and finally the gas goes through a methanation step to remove residual traces of carbon oxides.
In large plants, the flue gas and reformed gas boilers are separate units but have a common steam system, whereas in small hydrogen plants these boilers can be combined into a single module. The flue gas boiler is a water tube unit; the reformed and converted gas boilers are fire tube units connected to the same steam drum. The flue gas boiler contains various heating surfaces such as the feed
|
FIgure 2.11 Reformed gas boiler with internal gas bypass system. (Courtesy of ABCO Industries, Abilene, TX.) |
Preheat coil, evaporator, superheater, economizer, and air heater. The casing is refractory-lined, and extended surfaces are used where feasible because the gas stream is generally clean. The steam generated in the reformed gas boiler is often combined with the saturated steam generated in the flue gas boiler and then superheated in the superheater of the flue gas boiler. This is a substantial quantity of steam (often referred to as import steam), so the performance of the superheater must be checked for cases when the import steam quantity diminishes or is reduced to zero for various reasons.
The reformed gas boiler, which handles gases containing a large volume of hydrogen and water at high pressure, operates at high heat flux; the heat transfer coefficient with reformed gases is about 6-8 times higher than those of typical flue gases from combustion of natural gas; see Q8.64. Hence the heat flux at the inlet to the reformed gas boiler is limited to less than 100,000 Btu/ft2h to minimize concerns about vapor formation over the tubes and possible departure from nucleate boiling conditions (DNB). The gas properties for typical reformed gas and flue gases are listed in Table 8.45 (Chap. 8). The higher thermal conductivity and specific heat and lower viscosity coupled with higher mass flow per tube leads to higher heat transfer rates and hence higher heat flux in reformed gas boilers. Note that the heat transfer coefficient is proportional to
FspedfcheatV’4x (thermal conductivity)»’6
Viscosity
As discussed in Q8.02.
Generally fire tube boilers are ideal for high gas pressures, though a few European suppliers have built water tube designs for this application.



 18 июля, 2013
18 июля, 2013  doctype
doctype 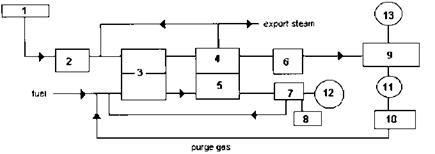

 Опубликовано в рубрике
Опубликовано в рубрике