A sulfur plant forms an important part of a gas processing system in a refinery. Sulfur is present in natural gas as hydrogen sulfide (H2S); it is the by-product of processing natural gas and refining high sulfur crude oils. For process and combustion applications, the sulfur in the natural gas has to be removed. Sulfur recovery refers to the conversion of hydrogen sulfide to elemental sulfur. The most common process for sulfur removal is the Claus process, which recovers about 95-97% of the hydrogen sulfide in the feedstream. Waste heat boilers are an important part of this process (Fig. 2.4).
The Claus process used today is a modification of a process first used in 1883, in which H2S was reacted over a catalyst with air to form elemental sulfur and water. The reaction is expressed as
H2S + 1/2O2 -! S + H2O
Control of this exothermic reaction was difficult, and sulfur recovery efficiency was low. Modifications later included burning one third of the H2S to produce sulfur dioxide, SO2, which is reacted with the remaining H2S to produce elemental sulfur. This process consists of multistage catalytic oxidation of hydrogen sulfide according to the reactions
2H2S + 3O2 ! 2SO2 + 2H2O + heat 2H2S + O2 ! 2S + 2H2O
Each catalytic stage consists of a gas reheater, a catalyst chamber, and a condenser as shown in Fig. 2.4.
|
FIgure 2.4 Claus process for sulfur recovery. |
In addition to the oxidation of H2S to SO2 and the reaction of SO2 with H2S in the reaction furnace, many other side reactions occur, such as
CO2 +H2S! COS + H2O
COS + H2S! CS2 + H2O
2COS! CO2 + CS2
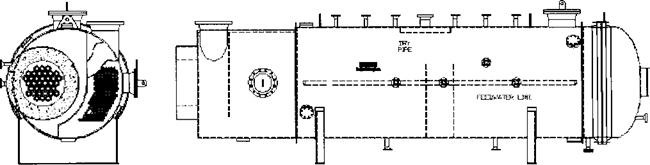
The gas stream contains CO2, H2S, SO2, H2, CH4, and water vapor in addition to various species of sulfur. The duty of the boiler behind the sulfur combustor includes both sensible heat from cooling of the gas stream from 2600°F to about 650°F and the duty associated with the transformation of various species of sulfur. The reaction furnace normally operates at 1800-2800°F, and the flue gases are cooled in a waste heat boiler (Fig. 2.5), in which saturated steam at about 600psig is generated. This is typically of two-gas-pass design, though single-pass designs have been used. The gas is cooled to about 1200°F in the first pass and finally to about 650°F in the two-pass boiler.
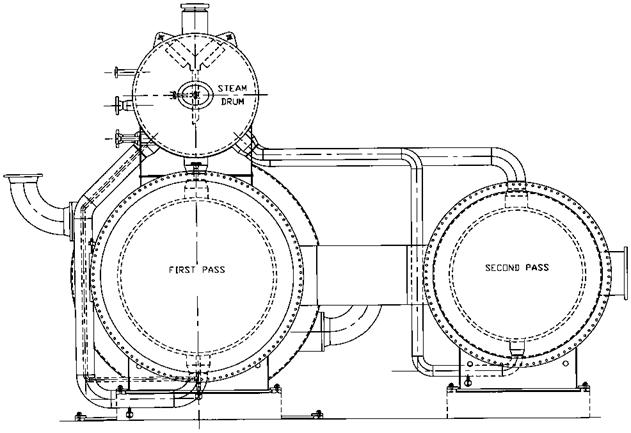
Figure 2.6 Shows the boiler for a large sulfur recovery plant, which consists of two separate shells for each pass connected to a common steam drum. The steam drum is external to the boiler. The external downcomer and riser system ensures adequate cooling of the tubes and the tube sheet, which is refractory — lined; ferrules are also used for further protection of the tube sheet. Ferrules are generally made of ceramic material and are used to transfer the heat from the hot flue gases (at about 2800°F) to the tubes, which are cooled by water. The refractory on the tube sheet, which is about 4 in. thick and made of a high grade, high density castable, lowers the tube sheet temperature at the hot end and thus limits the thermal stress across it. The inlet gas chamber is also refractory-lined. The casing is kept above 350-400°F through a combination of internal and external insulation to minimize concerns regarding acid dew point corrosion. This is often referred to as ‘‘hot casing.’’ Q8.56 discusses this concept. The exit gas chamber is externally insulated, as are also the drum, downcomer, riser pipes, and exchanger. The high pressure saturated steam, which is generated at about 600- 650psig, is purified by using steam drum internals and sent for process use. About 65-70% of the sulfur is removed in the boiler as liquid sulfur by using heated drains.
Though the boiler generally operates above the sulfur dew point, some sulfur may condense at partial loads and during transient start-up or shutdown mode. The cooled gases exiting the exchanger are reheated to maintain acceptable reaction rates and to ensure that process gases remain above the sulfur dew point and are sent to the catalyst beds for further conversion as shown in Fig. 2.4. The catalytic reactors using alumina or bauxite catalysts operate at lower temperatures, ranging from 200 to 315°C. Because this reaction represents an equilibrium chemical reaction, it is not possible for a Claus plant to convert all of the
|
FIgure 2.5 Waste heat boiler for sulfur recovery plant. (Courtesy of ABCO Industries, Abilene, TX.) |
|
FIgure 2.6 Multiple boiler passes connected to a common steam drum. (Courtesy of ABCO Industries, Abilene, TX.) |
|
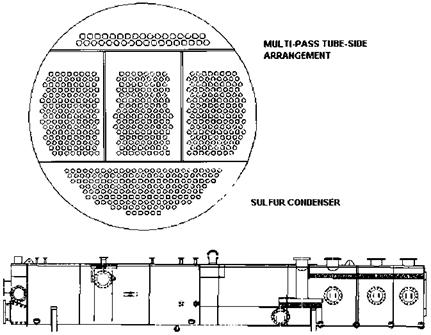
Figure 2.7 Sulfur condenser. (Courtesy of ABCO Industries, Abilene, TX.) |
Incoming sulfur to elemental sulfur. Therefore two or more stages are used. Each catalytic stage can recover one half to two-thirds of the incoming sulfur. Acid gas is also introduced at each catalyst stage as shown. The gas stream from each stage is cooled in another low pressure boiler, called the sulfur condenser, which condenses some of the sulfur. These gas streams generate low pressure steam at about 50-70 psig in the sulfur condenser.
If the flue gas quantity is small, a single-shell fire tube boiler handles all the streams from the reactors (Fig. 2.7). Each stage has its own gas inlet and exit connections. The outlet gas temperatures of these exchangers are around 330- 360°F. From the condenser of the final catalytic stage the process stream passes on to some form of tail gas treatment process. The tail gas contains H2S, SO2, sulfur vapor, and traces of other sulfur compounds and is further treated downstream and vented.



 18 июля, 2013
18 июля, 2013  doctype
doctype 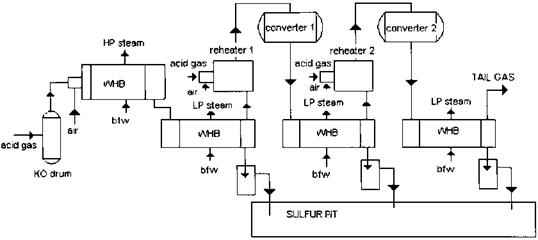
 Опубликовано в рубрике
Опубликовано в рубрике