Before going into further details of how the boiler or HRSG is impacted by emission regulations, one should first understand what the various pollutants are
|
Figure 4.3 Options for NOx removal in boilers and HRSGs. |
And how they are formed. In the process of combustion of fossil fuels, be it in steam generators, gas turbines, or engines, several pollutants are released to the environment. These include carbon dioxide (CO2), oxides of nitrogen (NOx), carbon monoxide (CO), oxides of sulfur (SOx), and volatile organic compounds (VOCs).
Carbon dioxide is considered to be responsible for the greenhouse effect and global warming. Concentrations of 3-6% can cause headaches; larger concentrations can lead to unconsciousness and possibly death. Coal generates about 200 lb CO2/MM Btu fired; oil generates 150 lb and natural gas about 100 lb per MM Btu. Hence one can see why natural gas is the preferred fuel in any fired equipment. CO2 molecules retain infrared heat energy, preventing normal radiation from the earth and leading to warming of the atmosphere. There are several processes, such as amine-based systems that can remove CO2 from flue gas streams, but these can be justified only in large plants.
The presence of carbon monoxide (CO) in flue gases is indicative of inefficient combustion and may be due to poor burner operation, improper settings, or even poor boiler design. CO is dangerous to the health of humans and other living creatures. It passes through the lungs directly into the bloodstream, where it reduces the ability of the red blood cells to carry oxygen. It can cause fainting and even death. At an exposure of only 0.1% by volume (1000 ppm) in air, a human being will be comatose in less than 2h. A few regulations establish a maximum exposure of CO of 9 ppm for an 8 h average and 13 ppm for any 1 h period.
Oxides of nitrogen, NOx, are predominantly NO and NO2. The majority of NOx produced during combustion is NO (95%). NOx is responsible for the formation of ground-level ozone or smog. Oxides of sulfur, SOx, are formed when fuels containing sulfur are fired. Sulfur dioxide (SO2) and sulfur trioxide (SO3) are responsible for acid rain and can damage plant life and materials of construction. The Taj Mahal in India is a good example of what acid formation from nearby refineries emitting oxides of sulfur can do to the luster and beauty of marble over a period of time. Particulates are also formed during combustion that disperse in the air to form haze and smog, affecting visibility. Dangerous driving conditions are created in some places due to smog formation. Inhalation of particulates affects the lungs and the digestive system.
Volatile organic compounds (VOCs), which are generated in industrial processes such as those of chemical and petrochemical plants, also cause harmful ozone.
Tremendous efforts are being made to reduce these pollutants in power and process plants, refinery heaters, and combustion equipment.



 5 августа, 2013
5 августа, 2013  doctype
doctype 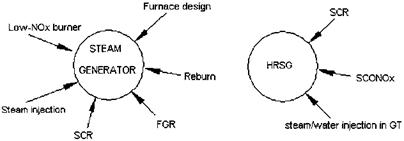
 Опубликовано в рубрике
Опубликовано в рубрике