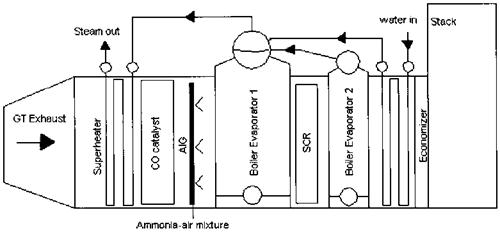
If the desired CO and NOx levels are very low, on the order of single digits, a selective catalytic reduction (SCR) system may have to be used in boilers and HRSGs, Because most catalysts operate efficiently within a temperature window, generally 650-780°F, the boiler should have a gas bypass system to accommodate the gas temperature window at all loads. One can see from Fig. 4.5 How the gas temperature profile across a packaged boiler varies with load. As the load decreases, the gas temperature at the various surfaces decreases because a smaller amount of flue gases is generated at lower load. Hence a gas bypass system, as shown iN Fig. 4.6, that mixes the hot flue gases taken from the convection bank with the cooler gases at the evaporator exit ensures a higher gas temperature at the SCR at low loads. Heat recovery steam generators (HRSGs) also use the SCR system to limit NOx, and, again, to match the gas temperature window of 650- 780°F the evaporator is often split up as shown in Fig. 4.7. If we did not split up the evaporator, we would have a very low gas temperature at its exit; also we cannot locate the SCR system ahead of the evaporator, because the gas
|
FIgure 4.6 Gas bypass system in boiler using (a) FGR and (b) SCR methods for NOx control. |
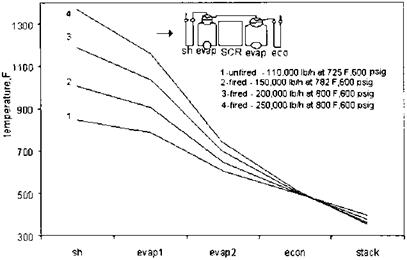
Temperature there is very high. As shown in Fig. 4.7, the two evaporator circuits are in parallel. External downcomers and risers are used to ensure adequate circulation through both the evaporator modules. Figure 4.8 Shows the gas temperatures entering various sections of a fired HRSG—superheater, evaporator, economizer, and stack—at various steam flows. The gas temperature at the entrance of the second-stage evaporator section may be seen to be in the range of 650-800°F. The SCR system adds about 3-4 in. WC to the boiler or HRSG gas- side pressure drop, which is an operating expense as discussed earlier.
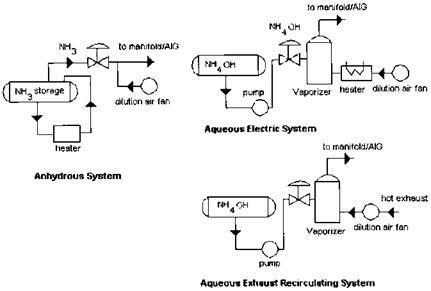
The selective catalytic reduction (SCR) method uses the same reaction process as SNCR except that a catalyst is employed to lower the temperature of operation and also increase the efficiency of conversion. Ammonia or urea is used in these reactions as the reagent. Figure 4.9 shows how ammonia is added in three different systems. The most common method uses anhydrous ammonia, which is pure ammonia. Anhydrous ammonia is toxic and hazardous, particularly if the neighborhood has a large population. It has a high vapor pressure at ordinary temperatures and thus requires thick shells for the storage tanks. Its release to the atmosphere can cause environmental problems, and extreme caution is required to handle such a situation. However, this is the least expensive way to feed ammonia into the HRSG.
|
FIgure 4.7 HRSG showing location of NOx (SCR) and CO catalysts. |
|
FIgure 4.8 HRSG gas temperature profiles as a function of steam generation. sh, superheater; econ, economizer; evap, evaporator. |
|
Figure 4.9 Ammonia injection methods. (Courtesy of Peerless Manufacturing, Dallas, TX.) |
Aqueous NH3(NH4OH), which is a mixture of ammonia and water, is safer to handle. A typical grade contains 30% ammonia and 70% water. It has nearly atmospheric vapor pressure at ordinary temperatures. The liquid ammonia is pumped to a vaporizer and mixed with heated air before being sent to the mixing grid. Urea systems, which generate ammonia on-site, are also safer and have been recently introduced. Dry urea is dissolved to form an aqueous solution, which is fed to an in-line reactor to generate ammonia by hydrolysis. Heat is applied to carry out the reactions under controlled conditions. The ammonia is mixed with air and then injected through a grid into the gas stream.
Computational fluid dynamics (CFD) analysis is done to ensure that the gas velocity distribution across the boiler or HRSG cross section is uniform, with variations within 15%. The ammonia vapor is mixed with air and sprayed into the flue gas stream at the desired location before coming into contact with the catalyst. A heat transfer surface located immediately behind the ammonia injection grid ensures good mixing of ammonia vapor with the flue gases. The optimum gas temperature for the NOx reduction reactions with most catalysts is 600-780°F as mentioned earlier. Below this temperature, chemical reactivity is impaired, and above it physical damage can occur to the catalyst through sintering. From the boiler or HRSG design viewpoint, a suitable location has to be found for the SCR so that at the wide range of loads, the temperature window is maintained, to ensure that undesirable oxidation of ammonia to NO does not take place. This is accomplished in a boiler by using a gas bypass system as discussed earlier. The ammonia injection system is located upstream of the SCR and should have sufficient mixing length that the flue gases can react with ammonia. SCR efficiency ratings are in excess of 90%. A gas pressure drop across the catalyst of about 3-4 in. WC adds to the fan power consumption in a steam generator and could be a significant power decrement in a gas turbine plant. Catalysts are typically platinum, vanadium, tungsten, and noble metals and zeolites, which are used at higher temperatures.
Typical reactions are
Catalyst
4NH3 + 4NO + O2 -4 4N2 + 6H2O
Catalyst
4NH3 + 2NO2 —y 3N2 + 6H2O
To complete these reactions, slightly more NH3 than required is injected into the gas stream. This excess ammonia, which is called slip, is generally limited to a single-digit value (less than 5 ppm), through a control and emission monitoring system. The slip value increases gradually over a period of time as the catalyst nears the end of its service life.
Sulfur-containing flue gas streams present problems for boilers and HRSGs. The presence of vanadium in the SCR converts SO2 to SO3, which can react with excess ammonia to form ammonium sulfate or with water vapor to form sulfuric acid, causing problems such as fouling and plugging of tubes downstream of the boiler or HRSG. Distillate oil contains a small amount of sulfur, hence the only way to minimize this concern is to limit the operating hours on oil fuels. Lowering the ammonia slip also helps, but this can lower the NOx reduction efficiency.
Environmentally ammonium sulfate and bisulfate are particulates that contribute to visible haze and acidify lakes and ground areas when they settle out of the air.
Sulfates are formed according to the equations
SO3 + NH3 + H2O! NH4HSO4
SO2 + 2NH3 + H2O! (NH4)2SO4
Ammonium sulfate is a sticky substance that can be deposited on heat transfer surfaces and cause fouling. The gas pressure drop across the heating surfaces also increases over a period of time. If the ammonia slip is less than 10 ppm and the SO3 concentration is less than 5 ppm, expert opinion is that the probability of ammonium sulfate formation is practically nil unless the gas temperature is low, on the order of 200°C. Hence low gas temperatures should be avoided, particularly at the catalysts, because salt formation and deposits there would be detrimental to the life of the catalyst. Some suppliers require a minimum of 450- 500°F at the catalyst to minimize these reactions. Either ammonium sulfate or ammonium bisulfate will be formed by the reaction of SO3 and excess ammonia downstream of the SCR catalyst. In general, ammonium sulfate is considerably less corrosive than ammonium bisulfate.
One should keep the boiler or HRSG warm in standby conditions during brief shutdowns if fuel oils are fired. Shutdown and isolation of the HRSG after oil firing should be avoided because the SO3 can condense during the cooling phase. For boilers or HRSGs firing natural gas fuels, fortunately, there are no such concerns as those just discussed. It may be noted that the presence of water vapor in the flue gases has an adverse effect on NOx reduction efficiency.
Selective catalytic reduction systems have efficiencies of 90-95%. However, they are expensive and may cost from $3000 to $5000/MM Btu/h in gas or oil-fired packaged boilers. For gas turbines the cost could range from $40 to 100/kW. In some coal-fired plants where regenerative air heaters are used, the hot end heating elements are coated with a catalyst material to convert NOx to N2 and H2O.
The SCONOx system is a recent development that is claimed to reduce NOx and CO levels to 2-5 ppmv with a single catalyst. It does not use ammonia or urea and hence avoids the concerns associated with handling ammonia. The system can operate efficiently at 300-700°F, which is an advantage because the HRSG evaporator need not be split up. Typically the gas temperature between the evaporator and economizer of an HRSG is in this range. Dampers are not needed to control the gas temperature in steam generators at low loads. This method has been used in a few HRSGs but not in packaged boilers.
The SCONOx catalyst works by simultaneously oxidizing CO to CO2, hydrocarbons to CO2 + H2O, and NOx to NO2 and then absorbing NO2 onto its platinum surface through the use of a potassium carbonate absorber coating. These reactions, shown below, are referred to as the ‘‘oxidation/absorption cycle.’’
CO + |O2 4 CO2
NO + lO2 4 NO2
CH2O + O2 4 CO2 + H2O
2NO2 + K2CO3 4 CO2 + KNO2 + KNO3
The CO2 produced by these reactions is exhausted up the stack. The potassium carbonate coating reacts to form potassium nitrates and nitrites, which remain on the surface of the catalyst.
The SCONOx catalyst can be compared to a sponge absorbing water. It becomes saturated with NOx and must be regenerated. When all of the carbonate absorber coating on the catalyst surface has reacted to form nitrogen compounds, NOx will no longer be absorbed, and the catalyst must enter the regeneration cycle.
The unique regeneration cycle is accomplished by passing a dilute hydrogen reducing gas across the surface of the catalyst in the absence of oxygen. The hydrogen reacts with nitrites and nitrates to form water and elemental nitrogen. Carbon dioxide in the regeneration gas reacts with potassium nitrites and nitrates to form potassium carbonate, which is the absorber coating that was on the catalyst surface before the oxidation/absorption cycle began. This cycle is called the ‘‘regeneration cycle.’’
KNO2 + KNO3 + 4H2 + CO2 4 K2CO3 + 4H2O + N2
Water and elemental nitrogen are exhausted up the stack instead of NOx, and potassium carbonate is once again present on the catalyst surface, allowing the entire cycle to begin again.
Because the regeneration cycle must take place in an oxygen-free environment, a section of catalyst undergoing regeneration must be isolated from the exhaust gases, usually by a set of louvers, one upstream of the section being regenerated and one downstream. During the regeneration cycle, these louvers close and a valve allows the regeneration gas into the section. Stainless steel strips on the louvers minimize leaks during operation. A SCONOx system has five to 15 sections of catalyst, depending on gas flow, design, etc. At any given time, 80% of the sections are in the oxidation/absorption cycle and 20% are in the regeneration mode. Because the same number of sections are always in the regeneration mode, the production of regeneration gas proceeds at a constant rate. A regeneration cycle lasts for 3-5 min, so each section is in oxidation/absorption mode for 9-15min.
The SCONOx technology is still being developed and have yet to accumulate significant operational experience compared to the SCR system. It is also very expensive and is sensitive to sulfur, even the small amount in natural gas. For a 2.5ppmv NOx limit from a 501°F Westinghouse gas turbine, studies show that the cost of SCONOx is more than that of the SCR system. However, with technological improvements, it could become an economically viable option.



 6 августа, 2013
6 августа, 2013  doctype
doctype 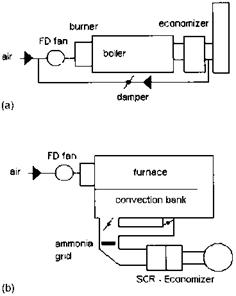
 Опубликовано в рубрике
Опубликовано в рубрике