Coals are complex substances that are geologically formed from ancient vegetation by the combination of time, pressure, and heat of the earth over several millennia. Depending on how long the vegetable matter has been subjected to these conditions, the resulting coals assume several properties. The most ancient coals under higher pressure would have converted practically all the vegetable matter into fixed carbon (FC), whereas the youngest coals with lesser pressure are still substantially woody and contain much more volatile matter (VM) than FC. Ash is the sand trapped along with wood in the formation of coal.
Classification of Coals
There are several classifications for coals. In the United States, they are classified by rank as per ASTM D388. Rank is the general measure of degree of coalification. In Europe, there are separate classifications for hard coals and brown coals. Hard coals have gross
|
TABLE 3.1 Coal Classification
|
|
Source: Adapted from ASTM D388. |
Calorific value (GCV) greater than —23.86 MJ/kg, 5,700 kcal/kg, or 10,260 Btu/lb on a dry ash-free (daf) basis. A broad classification of coals based on ASTM D388 is given in Table 3.1.
• The properties used for ranking of coals are GCV, M, VM, and agglomerating character.
• Older coals are classified by dry mineral matter free (dmmf) carbon and younger coals by moist mmf GCV.
• Parr’s formulae are followed for calculating the mmf C, VM, and GCV using proximate analysis (PA). The moist condition in low-rank fuels refers to the bed, inherent, or equilibrium moisture.
• Agglomerating or caking nature of coals is the swelling property (explained later) and determined by ASTM D720.
These data are presented in graphical form in Figure 3.1. Certain interesting trends can be observed.
• With increasing rank, FC and GCV increase whereas M and VM decrease.
• The ratio of FC to VM is called fuel ratio, which increases with rank.
• The higher the fuel ratio, the higher the GCV. But with anthracites, the GCV, in fact, drops, as there is not enough VM to contribute to the heat value.
• Lower ranks have higher VM and O2 contents, which aid ignition and enhance combustibility and flame stability.
• Low-rank coals produce self-pulverization during combustion as the inherent moisture locked in the pores is heated and expands rapidly (volume expansion of 1:1600) to fragment the fuel particles.
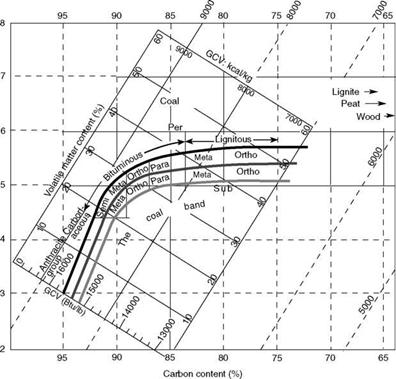
In the United Kingdom, Seyler’s classification forms the basis on which the analytical data on daf are plotted. Most coals fall in a band in which daf VM and daf GCV are plotted on
|
|
|
80 |
|
60 |
|
20 |
|
10000 18000 41.9 MJ/kg 8000 14400 33.5 Btu/lb 6000 10800 25.1 Kcal/kg 4000 7200 16.7 |
|
Fixed carbon |
|
FIGURE 3.1 Proximate analyses and GCV of various classes of coals. |
|
S. 40 |
|
FIGURE 3.2 Seyler’s classification of coal. (From Her Majesty’s Stationery Office, U. K. With permission.) |
X — and Y-axes. When daf C and H are also plotted on the two axes, there emerges a comprehensive and reliable chart of coal properties (Figure 3.2).
Analysis of Coals
The coal analysis is of two types. Both types are important, have different purposes, and are complementary.
Proximate Analysis This analysis tells us how the coal burns. It assesses four items:
1. Moisture (M)—the total moisture consisting of inherent/interstitial and superficial/surface moisture
2. Ash (A)—the residue left on burning of the coal
3. Volatile matter—the gaseous portion that burns above bed in free space
4. Fixed carbon—the solid portion of fuel that burns on the grate or in the bed
Moisture and ash together are known as the burden or ballast as they do not contribute to the heating value (HV).
1. Moisture. Total moisture of as-mined coal varies a great deal. It is about
• 5% in low-volatile bituminous coal
• 12% in high-volatile bituminous coal
• 35-65% in lignites
On burning, the moisture cools the flue gases, and hence reduces the tendency to form fuel NOx. It also increases the volume of gases.
I. Inherent moisture is trapped inside the fuel and is progressively released along with VM up to a high temperature of —700°C. This moisture exists as a quality of the seam in its natural state.
Ii. Surface moisture is picked up by coal due to its hygroscopic nature from the surroundings due to
• Humidity in air
• Rain or snow exposure during transportation
• Washing for ash reduction
• Mining operations
A. Moisture in the as-received coal is the total moisture consisting of surface and inherent moisture.
I. The visible surface moisture or free moisture dries away and reaches equilibrium with the ambient humidity when left exposed to air for some time. This is known as air-dried condition of fuel. The moisture left in coal after air drying is the remaining surface moisture. It leaves the coal quickly on heating to —105°C. Water held superficially increases as the coal size reduces.
Ii. When the sample is further heated in the oven to —700 to 750°C, the inherent moisture also leaves the coal.
Figure 3.3 shows the different types of moisture at different stages of drying. The air-dried condition is normally used for design purposes.
2. Ash is the incombustible mineral matter (MM) left behind when coal is burnt.
In the laboratory, it is the residue left on complete combustion at 700-750°C in a muffle furnace performed in a carefully controlled and specified manner. Ash is of two types—adventitious and inherent.
A. Adventitious ash is the material derived from shale, clay, pyrites, and dirt from earthy or stony bands in the coal seam.
Free moisture
Inherent moisture
|
Ash |
![]() Mineral
Mineral
Matter
|
■0 (D > (D O (D |
![]() Volatile matter
Volatile matter
|
(D > Ы |
|
(Ц ЈE "CЦ (D C Јz Ы |
|
(Ц Ы |
|
CЦ O O |
|
|
FIGURE 3.3
Bases of coal analysis. (From Her Majesty’s Stationery Office, U. K. With permission.)
B. Inherent ash is the mineral substance derived from vegetable matter.
Ash is composed of compounds of Si, Al, Fe, and Ca and, to a lesser extent, compounds of Mg, Ti, Na, and K. Although they are reported as oxides in analysis, they occur in ash as a mixture of silicates, oxides, sulfates, and so on. Ash forms nearly 90% of the MM, which additionally accounts for
A. The combined moisture and CO2 expelled from clays and other minerals by heat
B. The difference of weight between the original MM and the substance formed due to combustion
3. Volatile matter is a complex mixture of organic materials, which volatilizes quickly on heating at —300°C and burns in suspension in a furnace. The higher the VM, the lower the ignition temperature and greater the combustion speed—it is easier to ignite and burn the coal. Coals with less than —15% VM on a daf basis are termed low volatile. Their ignition requires higher temperature and burning is slow and hence requires a larger furnace volume.
Volatile matter is ascertained as the percentage loss in weight on heating a gram of coal with no air under standard conditions in a crucible for 7 min at 950°C. Moisture is expelled at the same time but recorded separately.
Expelled VM contains the following:
A. Water resulting from the combustion of H2
B. Complex mixtures of H2, O2, CO, CH4, C2H6, and other hydrocarbons (HCs)
C. Tar, a mixture of HCs and organic compounds especially phenols
No VM exists as such in coal but is formed chemically on the destruction of coal. Inerts and noncombustible matter form 4% of the total VM in low-volatile coals to 40% in subbituminous coals.
4. Fixed carbon is not the carbon obtained in ultimate analysis (UA). It is the carbonaceous residue left in the crucible after driving away the VM. It is not the
Total carbon, as total carbon is distributed between FC and VM. It is also not the pure carbon, as it contains traces of H, O, and N and about half the sulfur.
Fixed carbon needs to be burnt in solid state either on the grate or in the bed in fluidized bed combustion (FBC) or as solid particles in pulverized fuel (PF) firing.
FC decides the burning equipment. Fixed carbon is not measured but deduced by subtracting (M + A + VM) from 100.
Bases of Reporting Analysis There are several ways of reporting the analysis and the basis should be made clear.
• As-received basis is the analysis of coal as received at the plant and delivered for analysis.
• Air-dried basis. Coal is always analyzed in this condition. It is ground to pass through a 0.2 mm screen and spread out in a thin layer and allowed to dry until the retained moisture is in equilibrium with the ambient humidity. Then the inherent moisture, VM, and ash are determined and FC is calculated by the difference.
• Dry basis. Air-dried VM, FC, and ash are multiplied by 100/100 — IM, where IM is the inherent moisture.
• daf basis. Air-dried VM and FC are multiplied by 100/100 — IM-A, where A is ash.
• Dry MM free basis. Air-dried VM and FC are multiplied by 100/100 — MM — IM.
Figure 3.3 clarifies these different terms.
Ultimate Analysis This is the chemical analysis of the fuel in which carbon, hydrogen, nitrogen, sulfur, and oxygen (by difference) are determined for their elemental weights in the laboratory by prescribed methods. The UA is useful for the calculation of air and gas quantities and other combustion calculations.
• Elemental carbon is the sum of carbon in FC and VM. The CO2 in the flue gas is the result of this carbon.
• All the hydrogen in the fuel produces water nine times its weight on combustion. Together with water in the fuel, the total represents the moisture in flue gas.
• Nitrogen as inert material does not participate in combustion. At high temperatures it combines with O2 to produce traces of fuel NOx.
• Sulfur in coal occurs in three forms:
I. Organic combination
Ii. Sulfide ion as FeS2 in pyrite or FeSx as marcasite crystals
Iii. Sulfate ion as CaSO4 or Fe2SO4
Sulfur on combustion forms SO2 and partly SO3 and the latter in combination with water forms sulfurous acid, which produces gas side corrosion in locations that cool flue gases below the acid dew point.
• Oxygen in fuel is indicative of the rank; higher O2 equals lower rank. O2 represents a loss of fuel potential as it combines with C and H to form CO2 and H2O. It reduces the air requirement for combustion. High O2 and low HV go together. O2 in fuel is determined by the difference.
Calorific Value
Calorific value (CV) or HV is the heat content of the fuel expressed in megajoules per kilogram, kilocalories per kilogram, or British thermal units per pound. It can apply to coal as delivered, as fired, as dry, and as daf.
• There are two CVs in practice both representing different aspects. The GCV or higher CV (HCV) is more popular in the United States and United Kingdom, whereas net CV (NCV) or lower CV (LCV) is preferred on the Continent.
• Gross calorific value is the total HV of the fuel, that is, the heat released from the combustion of unit fuel quantity.
• Net calorific value is the heat available from GCV, that is, GCV less the heat in the water vapor.
• Water vapor is formed from the fuel hydrogen and the evaporation of fuel moisture, and it is in the flue gas at a partial pressure of —0.07 to 0.08 kg/cm2 in a superheated condition. The vapor leaves the boiler in the superheated condition only but without transferring its latent heat. Thus, the heat in water vapor of the flue gas is not available for heating and NCV recognizes this aspect.
NCV = GCV — (m + 9H2) X latent heat (3.1)
Where latent heat is used variously from —2.42 to 2.45 MJ/kg or 577 to 586 kcal/kg (1040-1055 Btu/lb).
• Gross calorific value is determined in the laboratory in a specified manner. A unit weight of pulverized coal is burnt in a water-submerged bomb calorimeter and the temperature rise of water is noted. The heat released or GCV is now known, as the water quantity is fixed. The NCV is deduced from GCV by applying the above-mentioned formula.
• For anthracite and bituminous coals, GCV can be calculated using Dulong’s formula from the UA, which is accurate within 2-3% of the results of the bomb calorimeter. The HV of the coal is due to the presence of C, H, O, and S and also due to heats of dissociation and other phenomena, which cannot be captured correctly, leading to the inaccuracy of the result of the formula.
GCV = 80.8C + 344.6(H — O/8) + 22.5S in kcal/kg
(3.2)
= 145.44C + 620.28(H — O/8) + 40.5S in Btu/lb where H, O, and S are percentages in UA.
Grindability Index
Grindability index indicates the ease of grinding the coal, a property that is vital for milling. It is an index showing the relative hardness of that particular coal compared with the standard soft coal with an index of 100.
The principle is that the work done in pulverizing is proportional to the new surface generated. The Hardgrove machine, named after the inventor, is a miniature pulverizer in which the amount of grinding energy can be measured. The method involves grinding a sample of 50 g of air-dried and —1.0 to 0.5 mm sieved test coal in the test mill for 60 revolutions and comparing the 75 |jm fraction with the standard curves. The pulverizer base capacities are rated on the Hardgrove index (HGI) of 50 or 55.
|
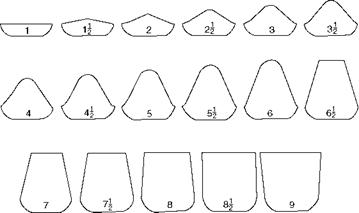
FIGURE 3.4 Coke profiles from swelling test. (Adapted from Her Majesty’s Stationery Office, U. K. With permission.) |
Caking and Coking Coals
Caking Coals or Agglomerating Coals These coals, when heated, quickly become plastic, fuse together, form large masses of semicoke, and disrupt the combustion process on a bed. This property is of importance to grate firing and bubbling fluidized bed combustion (BFBC) and not for PF and circulating fluidized bed combustion (CFBC). Caking property is worsened with more fines in fuel.
Caking or agglomerating is determined by specified tests in which the coal is heated till the M and VM are driven off and checked to determine whether the sample fuses together or remains as a powder. Depending on the cross-sectional area of the coke button formed, its free swelling index between 1 and 9 is determined (Figure 3.4). Free burning coals are nonagglomerating and the opposite of caking coals.
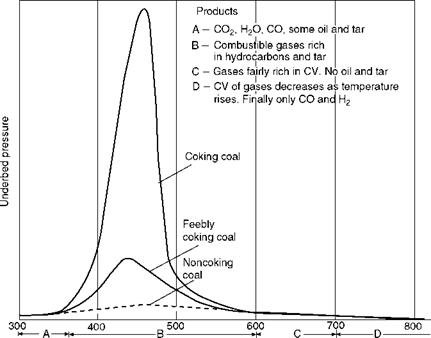
Coking Coals Coking coals make a good coke required for metallurgical purposes to produce high temperatures. When heated in coke ovens, out of contact with air, the coking coals lose their VM and form hard dense coke, consisting of FC and ash of the original coal. Good coking coals generally swell on heating, as reflected by the underbed pressure in Figure 3.5. This figure also captures the three phases of combustion—drying, devolatilization, and combustion of FC (also see Section 2.6.1). Coals that cake on a fuel bed do not necessarily make good coking coals. Subbituminous coals, lignites, and anthracites are noncaking coals.
Ignition Temperature
Combustible matter is a substance that has affinity for oxygen. There is a minimum temperature for each fuel and its constituents to enable the combustion reaction to take place, which is called the ignition temperature. This is the temperature beyond which more heat is produced in the reaction than what is lost to the surroundings to enable self-sustenance of the combustion. The ignition temperatures of the gases are higher than FC and vary widely but they distill off the fuel at a much lower temperature. Hence, the ignition temperature of coal is considered the same as that of FC (Table 3.2).
Coals Worldwide
The production of bituminous and subbituminous coals constitutes the maximum. These coals are spread across the globe with the United States, Russia, China, and Europe as the
|
TABLE 3.2 Approximate Ignition Temperatures for Various Combustibles
|
|
FIGURE 3.5 Behavior of coal on application of heat. (Adapted from Her Majesty’s Stationery Office, U. K. With permission.) |
![]()
|
Temperature (°C) |
![]() Leading producers. India, Australia, South Africa, and Indonesia are the other producers. Good-quality coals are abundant in the United States and Europe. India, China, and South Africa produce subbituminous coals with high ash content and low CV.
Leading producers. India, Australia, South Africa, and Indonesia are the other producers. Good-quality coals are abundant in the United States and Europe. India, China, and South Africa produce subbituminous coals with high ash content and low CV.
A list of typical steam coals that are suitable for use in power generation is given in Table 3.3 for ready reference. Coals supplied to power stations and for export may be
|
TABLE 3.3 Typical As-Received Analysis of Coals from Various Countries
|
|
Note: Conversions from MJ/kg to kcal/kg and Btu/lb have been rounded to the nearest five. |
At variance to the properties shown. They represent average properties as mined and received.
With the easing of trade norms, trading of coal has been on the rise. Traditionally, Australia, South Africa, and Indonesia have been among the leading exporters, and Japan, Europe, and South Korea have been among the leading importers. This circle of exporters and importers is enlarging.
Anthracites are the most ancient and fully formed coals, bordering on graphite on one end and bituminous coal on the other. They are dense, very hard, and brittle. They appear shiny black with no marks of layers. H2 and inherent moisture vary from 2.8 to 3.9% and
1.5 to 3.0%, respectively.
Most anthracites and semi anthracites have a lower GCV than the highest grade of bituminous coals. With very low VM (2-14% on a daf basis), consisting mainly of methane, the anthracites are difficult to burn.
They need very high temperatures (up to 600°C) to ignite. Burning on spreader stokers (SSs) is not possible but it is possible on chain grate stokers in sandwich mode along with coal (for ignition). Burning in PF mode is now common. It requires more grinding (80-85% through 75 |jm) and down-shot firing with W-shaped flames to give adequate time for the combustion of all the FC. Main uses of anthracites are in domestic heating, gas production, and power generation.
Meta anthracite, approaching graphite in structure and composition, burns with a short, smokeless blue flame. Being slow to ignite and difficult to burn, it has little commercial importance. Russia is a major producer of anthracite followed by China, North and South Korea, Western Europe, and the United States.
The word lignite comes from the Latin word lignum meaning wood. They are younger coals formed from plants rich in resin and hence high in volatiles and moisture. Lignite is a solid fuel that is more mature than peat and still shows a definitely woody structure.
Freshly mined lignite is tough and requires hammer blows to break down. But on exposure to air for a couple of days, part of the moisture evaporates and the lignite blocks disintegrate on slight pressure. Although it appears to be dry, lignite can contain about 30% moisture. Lignites with high moisture of 50-65% are called brown coals in Europe.
Lignites are lighter and more bulky; that is, the bulk density is less. Upon storage they lose their HV as the volatiles tend to leave the fuel. They are more prone to spontaneous combustion due to high oxygen and volatiles. It is not desirable, therefore, to have large stock piles. The storage bunkers are also sized for shorter duration.
Lignites with —35% moisture can be ground in pulverizers with high inlet air temperatures. But beater mills are required for brown coals, and gases from the boiler furnace are drawn for fuel drying. HGI has no relevance for brown coals. Lignites can be burnt on chain grates and SSs as well as in both BFBC and CFBC. High alkalies in ash tend to lower the ash fusion temperatures that can cause slagging and fouling. In BFBC, there are chances of in-bed tube fouling and corrosion.
In a way lignite is the opposite of coal in terms of combustion—daf coal has about 60% FC and 40% VM, which is reversed in daf lignite. Lignite therefore ignites at a lower temperature and burns faster. The high O2 content of lignite reduces the need for combustion air, but the high moisture in fuel increases the flue gas production. The furnace volume has to be larger and more secondary air (SA) and tertiary air (TA) need to be supplied to burn the large amounts of VM.
Lignite is next only to coal in terms of production and power generation. East Europe produces nearly half of the world’s production, followed by Western Europe, Russia, Australia, Turkey, the United States, and India.
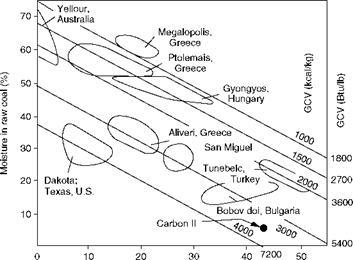
Lignite is widely spread across the globe. As the energy costs rise, the mining and burning of these inferior fuels become economically viable. The lignites vary a great deal in their composition and properties being younger coals. Figure 3.6 shows how the various lignites from different regions can be depicted as clusters with A, M, and CV considered. The higher the position of the mine in the graph, the younger the fuel, and more difficult it is to burn. The boilers and the handling equipment also become very large, and the initial investment in the power plant is higher. The running costs are usually lower due to the proximity of the power plant to the mine where the fuel is normally dug out of the open cast mines rather easily. Table 3.4 lists lignites worldwide for a quick reference.
Peat is the youngest of all the coals, still at an early stage of metamorphism of vegetable matter into coal. It is the product of partial decomposition, in the absence of air, of plant remains in marsh lands. Peat is a complex mixture of C, H, and O, which is low in S (—0.2%), N, and ash. Peat is yet to be fully exploited as a fuel in many countries where it is produced.
Peat is largely confined to higher latitudes of Northern countries such as the United States, Ireland, Russia, Sweden, and Finland. In the United States, it is the second most abundant fuel with extensive deposits after coal. Owing to low HV, peat cannot be transported over long distances economically. Russia, Finland, and Ireland have a number of peat-based power stations. In fact, in Sweden and Finland, peat and wood are the only indigenous fuels.
|
Ash in raw coal (%) FIGURE 3.6 Typical lignites of the world. (From Babcock and Wilcox Company, U. S.A. With permission.) |
|
Typical As-Received Analysis of Lignites
|
|
TABLE 3.5 TABLE 3.6 Typical Properties of Peat Typical Proximate Analysis on Dry Basis
|
|
TABLE 3.7 Typical Ultimate Analysis on daf Basis
|
Very high moisture levels are associated with peat. Undrained bog peat can have 80-95% moisture that reduces to —70 to 90% on draining and 35-55% on air drying. As fired, moisture must be <50% for a stable firing. The properties of peat vary considerably. A typical range of properties and analyses appears in Tables 3.5 through 3.7.
|
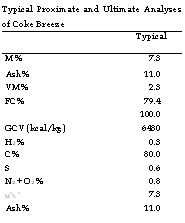
Typical Proximate and Ultimate Analyses of Coke Breeze
|
|
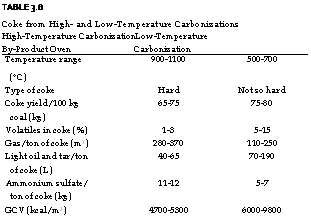
TABLE 3.8 Coke from High — and Low-Temperature Carbonizations High-Temperature Carbonization Low-Temperature By-Product Oven Carbonization
|
 Coke is devolatilized coking coal produced by heating coal in the absence of air in coke ovens. The VM and the moisture are driven out, leaving behind the FC, ash, and sulfur to
Coke is devolatilized coking coal produced by heating coal in the absence of air in coke ovens. The VM and the moisture are driven out, leaving behind the FC, ash, and sulfur to
Form a hard substance called coke. The process of decomposing coal into the gaseous and solid fractions is known as destructive distillation or carbonization. Only some coals with certain resinous substances can become plastic and fuse together to form coke. Depending on the coal properties, pressure and temperature employed, and the type of coke oven, the quality of coke varies.
High-temperature distillation is carried out between 900 and 1100°C in by-product ovens, whereas low-temperature carbonization is carried out between 500 and 700°C. At higher temperatures there is a greater evolution of gases, producing metallurgical and gas-making coke. Owing to the high FC, the coke produces a smokeless high-temperature burning, which is required in mostly blast furnaces and, to a much smaller extent, in pig iron cupolas. Low-temperature carbonization yields coke more suitable for domestic use whose production and consumption have declined over years.
About 5% of coke is undersized at <16 mm, which is unsuitable for metallurgical industry and is used mainly for steam generation. Coke breeze is very abrasive, as it contains higher ash than the rest of the coke. Since it has practically no VM, the ignition requires a high temperature.
Coke breeze is best fired in chain-grate stokers along with high-volatile coal in a sandwich manner. Erosion of firing equipment is also limited, as there are less moving parts. Erosion of pressure parts (PPs) is also considerably less, as there is no fuel in suspension. (Tables 3.8 and 3.9).
During petroleum cracking, the heavy residuals are processed further in a number of ways to produce lighter distillates, which leaves behind heavy residues such as delayed coke, fluid coke, and petroleum pitch, depending on the process adopted. These residues vary considerably.
• Delayed coking is the most widely adopted process, and the majority of the petroleum coke produced is delayed coke. It is called shot coke or sponge coke because of its appearance. Delayed coke appears like run-of-the mine coal but dull black.
|
TABLE 3.10 Ultimate Analysis of Delayed and Fluid Cokes
|
• Fluid coke is developed using FBC processes and resembles black sand. It consists of small hard spherical particles 200 |jm in diameter with very low VM (Table 3.10).
Petcoke has the following properties:
• High CV of 7800-8700 kcal/kg
• Low ash (<1%)
• High sulfur content of 2.9-9.8%
• Low VM
• High FC
Petcoke, despite its good CV, is difficult to ignite and burn due to very low VM and very high FC. Unburnt losses are quite high. Combustion-wise it is similar to high-FC, low-VM fuels, such as anthracite, but for its very high sulfur content. All the impurities of the original crude oil are concentrated in the petcoke as evidenced by very high sulfur and heavy metals in ash, such as vanadium and nickel. SO2 in excess of 13,000 mg/N m3 would result in flue gases when petcoke is burnt. Circulating fluidized bed combustion is best suited for burning this difficult fuel, as the desulfurization is easily performed, and recirculation of ash and fuel several times in a furnace ensures a high carbon burnup of >98%. The consumption of limestone for desulfurization, however, is very high, often as much as the fuel consumption.
Petcoke firing is finding a lot of resistance in many advanced countries and pollution norms are rather stringent. This is due to the high level of sulfur in the fuel, which contaminates the ground on which it is stored.
Bagasse
Bagasse is the waste product left after crushing of cane and extraction of juice in cane sugar mills. It is a seasonal product, as the crushing campaign lasts from 6 to 10 months in a year; the plant is longer near the equator and gets progressively shorter away from it. Cane is a tropical crop that extends across the globe. Bagasse forms 24-30% of the cane weight. Bagasse burning has been an integral part of the sugar cycle from the beginning. Steam and power requirements of these far-flung rural factories have been adequately met with bagasse-based cogeneration and off-season purchase of grid power. Traditionally the bagasse burning has been carried on inefficiently in sugar mills as there was always excess bagasse left with no great market value, and it is too bulky to transport or store. Burning was a way of disposal of this bulk. In the last couple of decades, there has been a sea change in this scenario with the enhanced possibilities of production of paper, certain value-added chemicals, and cogenerated power. Encouragement of its use for distributed power and green power even in small quantities has helped in adopting cogeneration in sugar factories in a big way. Bagasse has now attained its rightful place as a good, consistent, and bulk waste fuel in tropical countries that provides good market value. Bagasse burning is also environmentally friendly as combustion temperature is low due to the quenching effect of the fuel moisture and no fuel sulfur to pollute with sulfurous gases. It is now burnt efficiently in vastly improved boilers at increasingly higher pressures and temperatures at low NOX and SOX.
• It is a cellulosic and fibrous fuel with a PA of 45-52% M and 1-2% ash, having remarkably consistent properties across the continents varying by only ±2%.
• On a daf basis the VM is as high as 85-87%, which makes it highly inflammable on drying and vigorously burning. For the same reasons, the storage of bagasse in summers is fraught with twin problems of degradation of fuel due to loss of VM and fear of spontaneous combustion due to high oxygen. High VM makes burning of bagasse very easy and efficient in boilers.
• With small and uniform fibers, bagasse is easy to transport, distribute, spread, and burn, once its extremely low bulk density and fluffiness causing flow interruptions are properly understood and factored in.
• It is slightly soft and reasonably compressible, which makes it possible to bale and store or transport over some distance.
• With very little and low-density friable ash, there are practically no great problems of slagging and fouling in modern boilers having minimal refractory provided there is adequate furnace volume. In the brick set boilers with small furnaces this used to be a major problem with recurring clinker formation, as traces of sugar residue with low melting temperature would bond with refractory.
• Moisture is the most important aspect of bagasse, and it varies from mill to mill depending on the age, number of mills in tandem, milling process, and so on. Lower moisture improves fuel handling and combustion. Forty-five percent is more or less the optimum moisture that can be attained on a consistent basis with good, well-maintained milling tandem, and it rarely exceeds 52% even with the old mills. Efforts to reduce the fuel moisture to derive great benefits in fuel efficiency and reduced boiler and auxiliary sizes by heating with the outgoing boiler flue gases in bagasse driers have not been successful.
• Ash percentage is small and erosion can be kept under check by proper gas velocities. Use of mechanical harvesters brings a lot of unwanted mud with cane, making it abrasive due to extraneous silica. The inherent ash is light and fluffy.
• Bagasse-based cogeneration is gaining increasing popularity, and when connected to grid, it may be required to generate power even in off-season. The boilers will have to be multifuel to deal with this situation, usually using oil or coal as the auxiliary fuel.
• The ash-fusion temperature of bagasse ash is quite low. In multifuel firing, ashes from both fuels have a tendency to chemically interact to form a eutectic compound whose fusion temperature is lower than that of both the ashes. This leads to the problems of slagging and fouling that are not present when either fuel is fired alone. Slightly larger sizing of furnace volume and wider superheater (SH) tube spacing can eliminate the fouling problem. Analyses and properties of bagasse are given in Tables 3.11 and 3.12.
|
TABLE 3.11 Proximate and Ultimate Analyses of Bagasse on As-Received and daf Bases |
TABLE 3.12 Properties of Bagasse daf (%) |
|||
|
Daf |
C |
47.0 |
||
|
Range |
Basis |
H2 |
6.5 |
|
|
A (%) |
1.0-2.0 |
— |
O2 |
44.0 |
|
M (%) |
45-50 |
— |
N2 |
2.5 |
|
VM (%) |
42-44 |
85-87 |
S |
Traces |
|
FC (%) |
5-6 |
11-12 |
||
|
GCV (kcal/kg) |
2200-2300 |
4600 |
||
|
NCV (kcal/kg) |
1750-1850 |
4250 |
Rice husk is the protective cover on the rice seed, which is removed and rejected in the dehusking/dehulling operation of rice milling. Large amounts of rice husk are generated in the rice mills during the harvesting season. Husk forms between 20 and 25% of weight of dried rice.
• Rice husk is very uniform in size usually <3 mm, requiring no particular fuel preparation.
• Together with its CV of —3500 kcal/kg, rice husk is an attractive fuel except for its seasonality.
• It requires a lot of space for storage. It is normal, therefore, to adopt multifuel firing to take care of off-season. Captive power plants of 5-20 MW, attached to the rice mills, have been erected in a number of locations instead of transporting husk over longer distances.
• Rice husk has —15 to 20% ash and is highly abrasive, as the ash contains silica to the extent of 90%.
• It is very dry, containing only —7 to 9% moisture and not compressible for baling purposes.
• On a daf basis it has —20% FC and 80% VM, requiring a normal furnace volume and adequate SA. Husk demands a high ignition temperature and adequate time for combustion either on grate or on bed. VM leaves the fuel at —500°C.
Stoker firing is a good and simple firing system for rice husk. Its low fan power, simple operation, and seamless 1:4 turndown make it ideally suited for husk.
Bubbling fluidized bed combustion is also an alternative, as husk has a good 15-20% ash to create its bed material and is sufficiently heavy to stay in the bed, unlike a light material such as bagasse. The higher fan power is offset by better combustion efficiency of 2-4%. Both designs have to contend with abrasion issues. From an erosion standpoint, overbed BFBC appears better than underbed.
Silica in ash is mostly in crystalline form. Only —20% is in the amorphous form, and recovering silica in amorphous form has several high-end uses, which can be achieved only on controlled combustion of rice husk, limiting the firing temperatures to —860°C. For this reason, the husk is pulverized in special mills and burnt in suspension to obtain ash with the desired properties. Analyses and properties of rice husk are given in Tables 3.13 and 3.14.
|
Proximate and Ultimate Analyses of Rice Husk
|
|
TABLE 3.14 Properties of Rice Husk and Ash
|
Wood is a complex vegetable tissue composed mainly of carbohydrates, and in common with all types of vegetation, it has a relatively low HV in comparison with coal and oil. HV of different woods should have been nearly the same, but for the presence of varying amounts of resins, gums, and other substances, which creates a wide variation. For the same reason, any formula similar to Dulong for estimation of GCV does not work with wood.
Wood was the prime fuel till the early nineteenth century when coal and, later on, oil started displacing it. In the meanwhile the energy need and production has gone up dramatically. Progressive reduction of forests; better uses for wood, namely, furniture, paper, rayon, and so on; and enormous demand for energy that could be satisfied only by fossil fuels have combined to make wood and its products waste fuels today. In fact, wood-based steam generation is confined to Scandinavia, Canada, the United States, and certain South American countries, where forests are still abundant.
Hard and soft woods are the two broad categories, not based on any hardness measurement, but based on botanical terminology. Hard woods belong to trees with broad leaves and soft woods to trees with scale or needle-like leaves.
|
TABLE 3.16 Properties of Wood on daf Basis
Note: Resinous woods have slightly high CV. a GCV on daf basis. |

|
Ultimate Analysis of Wood on daf Basis
|
 In lumber manufacture, nearly 50% of the wood is turned into waste, with about
In lumber manufacture, nearly 50% of the wood is turned into waste, with about
• 20% in the form of slabs
• 10% as bark
• 20% as sawdust and shavings
Range of analysis of dry wood is given in Tables 3.15 and 3.16, covering both hard and soft woods.
• Moisture content of freshly cut wood varies from 30 to 50%, which reduces to 18-25% after a year of drying. There is a loss of fuel value in the meantime.
• Woods with <50% moisture burn well. Owing to rain, snow, or transportation by water, moisture content can go to as high as 70%. At more than —65% moisture, the combustion is not self-sustaining, as the heat produced is not sufficient to dry the moisture. Support fuel such as oil is then needed.
• Ash in wood is less at <2.5%.
• There is practically no nitrogen or sulfur, eliminating the fear of fuel NOX formation and corrosion.
• The moisture content depends on the type, handling, storage, and age of wood. Generally, wood logs can be taken as containing —40% moisture, sawdust, and chips 15-25%, and wood refuse from seasoned wood 15% moisture.
• The low HV and large bulk make it uneconomical to transport wood or wood waste over long distances, for example, more than 100 km.
Use of wood for steam generation is in the following three areas:
1. Wood producers, namely, saw mills using sawdust, shavings, bark, and other wood wastes
2. Wood users using wood chips
3. Paper mills using waste wet bark produced in the mill
Agrofuels, or biofuels, or vegetable fuels, as they are variously called, are essentially wastes generated by various crops. Fuels such as wood and bagasse are also agrofuels, but because of their relatively large availability, they have been used as regular fuels for a long time and generation of steam and power for a few decades now.
The distinction between agro/biofuels and biomass has to be clearly understood. Agrofuels form only a part of biomass, which is a comprehensive term embracing all organic matter formed, directly or indirectly, by virtue of photosynthesis. Besides agrofuels like crop, forest, agro-industrial residues and purpose-grown trees, biomass includes aquatic plants and even animal wastes.
Nature of Agrofuels
With increasing energy costs and growing concern toward the environment, there is a heightened interest in harnessing the various agrofuels, although the steam and power they generate on a stand-alone basis is rather modest as of now. The main drawback of agrofuels is their limited and seasonal availability coupled with limited transportability due to their bulky nature. However, with low or no S and very low N2, biofuels are ecologically friendly. The disadvantage turns to favor, as small power plants can be put up, distributed over a wide area and close to small communities provided they are built with fuel flexibility. If based entirely on agrofuels, the benefit of carbon credits also accrue.
Policy changes and financial incentives are being extended in many countries to make biofuel firing more acceptable and attractive. Agrowastes by their nature are
1. Bewildering in variety.
2. Variable in composition. The variation in properties is large from season to season, place to place, and lot to lot.
3. Seasonal. The campaigns for harvesting the crops last from a few weeks to a few months.
4. Limited. The quantities generated/harvested/collected are limited. Carting over longer distances is not economical.
5. Bulky and often fluffy. This makes long distance transport undesirable and in-plant movement and storage expensive.
6. Fast degrading. Being cellulosic in nature, they cannot be stored for long periods.
7. In need of support from a prime fuel to cover seasonality or shortfall in supplies. Alternatively the boilers are equipped to fire multiple agrofuels so that one or the other fuel can be burnt in each season.
Power plants of only 5-30 MW are popular from the view of fuel collection. Often fuels are cofired in limited quantities in large boilers.
Agrofuel Combustion
A look at the Seyler’s chart in Chapter 2 is necessary to gain a better understanding of combustion properties of agrofuels. General properties of agrofuels can be summarized and tabulated as shown in Table 3.17.
It is important to remember that no agrofuel can have, on a daf basis, a GCV <13,500 kcal/kg (7,500 Btu/lb) or air requirement <1.21 kg/1,000 kcal (6.8 lb/10,000 Btu) of GCV, which is the minimum for cellulose (C6H19O5).
Agrofuels are very friendly both from the ease of burning and from environmental compliance views. Stokers are the most appropriate firing equipment. Bubbling beds are also suitable. Since the bulk density is very low, many fuels are not suitable for BFBC as they cannot stay on bed and are carried away. Also, when the ash in fuels is very low, typically <10%,
General Range of Properties of Agrofuels
|
Range |
![]()
|
Remarks |
![]() Parameter
Parameter
|
72.0-90.0 5.6-5.8 -10.0 20.2-20.75 1.015-1.005 4500-5500 8000-10000 1.22/1000 6.85/10000 |
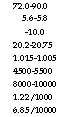
VM (daf) (%)
H2 (daf) (%)
M2(%)
Theoretical CO2 (%) f-factor
GCV (daf) (kcal/kg) GCV (daf) (Btu/lb) Theoretical air (kg/kcal) Theoretical air (lb/Btu)
For CO2 calculation 4000 ± 300 as received 7200 ± 550 as received Actual GCV
Bed ash replenishment can add to the cost of operations. But for fuels such as rice husk having a good percentage of ash, BFBC makes a good choice as the combustion efficiencies are higher and the boilers more compact if the tube erosion issues can be contained. Readers can refer to Chapter 11 on stoker firing for more details.
• With VM of 70-90% on a daf basis, agrofuels are efficient for both ignition and combustion.
• Negligible amounts of ash in fuel help in speeding up the burning of FC, thus enabling fast and easy burning of fuels with low unburnt loss.
• The ash-handling plant is smaller in size due to less ash produced, but has to be suitable to account for its low density.
• The fuel sizing can be a problem in fuels that are stringy.
• Biofuels are very light and often fluffy. This makes the fuel handling equipment larger.
• These fuels are rich in oxygen. This reduces the air requirement for combustion. Typical air requirement for coals and biofuels is given in the following table. On an average 8-10% less air is required.
|
Coal Biofuel |
![]() 1.31-1.36 kg/1,000 kcal 7.3-7.6 lb/10,000 Btu
1.31-1.36 kg/1,000 kcal 7.3-7.6 lb/10,000 Btu
1.22-1.24 kg/1,000 kcal 6.8-6.9 lb/10,000 Btu
• Flue gas generation is also lower except when there is high moisture in fuel, like in bagasse.
• In the furnace, as there is more combustion in suspension due to high VM, more SA needs to be supplied than for conventional fuels. Typically SA is —30% in stoker firing as against 20% for coal.
• An additional (third) row of air nozzles is needed to provide an air curtain in the upper furnace to prevent carry-over of fuel, besides providing air for combustion of volatiles. The carry-over tendency is due to the lower bulk and particle densities and also due to the fines generated in sizing or dehulling operation.
• The furnace exit gas temperature (FEGT) tends to be higher with biofuels than with coal, when both are burning on grates. This is because of greater heat release by VM and at higher levels in furnace compared with coal, which releases more heat close to the grate in burning the higher FC.
Of course, with bagasse the FEGT has to be lower as the gas is cooled by the high amount of moisture in fuel.
Table 3.18 provides combustion data for some minor biofuels. Calorific value is rounded to the nearest five.
Ash of Agrofuels
The main problem with the combustion of biofuels is the ash composition, which is highly variable among fuels as can be seen from Table 3.19. Although the ash in fuel is less,
|
TABLE 3.18 Combustion Properties of Minor Agrofuels
|
|
TABLE 3.19 Properties of Typical Select Agrofuels and Their Ash |
||||||||
|
Corn Stems |
Corn Cobs |
Corn Grain |
Straw |
Straw Pellets |
Rice Husk |
Wood Chips |
Bark |
|
|
Dimensions |
10 X 100 |
30 X 50 |
1-8 |
0.2-2.5 |
13 X 25 |
0.1-2.5 |
3 X 12 |
8 X 20 |
|
Particle density (kg/m3) |
360.00 |
385.00 |
420.00 |
650.00 |
1400.00 |
740.00 |
920.00 |
880.00 |
|
Bulk density (kg/m3) |
158.00 |
174.00 |
235.00 |
100.00 |
790.00 |
120.00 |
590.00 |
430.00 |
|
M (%) |
9.20 |
8.00 |
11.90 |
8.50 |
8.50 |
4.10 |
41.50 |
58.00 |
|
Proximate analysis: as-received |
||||||||
|
NCV (MJ/kg) |
15.80 |
16.30 |
15.20 |
16.6 |
16.50 |
12.90 |
9.55 |
6.52 |
|
Ash content (%) |
2.60 |
2.40 |
1.88 |
6.00 |
6.10 |
22.70 |
0.75 |
1.81 |
|
VM (%) |
67.30 |
69.10 |
75.00 |
61.60 |
61.40 |
59.70 |
47.94 |
31.79 |
|
FC (%) |
20.90 |
20.50 |
11.20 |
24.20 |
24.00 |
13.50 |
9.82 |
8.45 |
|
Ultimate analysis: (%) dry |
||||||||
|
Basis (wt) |
||||||||
|
C |
42.16 |
45.57 |
43.72 |
41.61 |
37.81 |
47.62 |
48.30 |
|
|
H2 |
6.28 |
5.93 |
7.18 |
6.70 |
4.68 |
6.11 |
6.34 |
|
|
O2 |
45.70 |
45.89 |
45.73 |
44.40 |
33.52 |
44.34 |
39.76 |
|
|
N2 |
0.71 |
0.74 |
1.24 |
0.66 |
0.28 |
0.65 |
1.29 |
|
|
S |
0.01 |
0.02 |
0.01 |
0.01 |
0.03 |
0.00 |
0.00 |
|
|
Ash composition (wt %) |
||||||||
|
SiO2 |
32.74 |
43.00 |
59.52 |
97.62 |
6.45 |
5.88 |
||
|
Al2O3 |
5.05 |
8.40 |
2.18 |
0.01 |
0.66 |
0.20 |
||
|
TiO2 |
0.02 |
0.40 |
0.02 |
0.01 |
0.02 |
0.02 |
||
|
Fe2O3 |
0.70 |
5.05 |
8.76 |
0.19 |
2.05 |
0.33 |
||
|
CaO |
2.63 |
5.10 |
6.52 |
0.01 |
73.05 |
80.72 |
||
|
MgO |
6.83 |
8.10 |
2.26 |
0.59 |
0.54 |
0.23 |
||
|
K2O |
29.24 |
15.30 |
13.16 |
0.87 |
4.50 |
2.57 |
||
|
N2a2O |
1.38 |
0.90 |
1.60 |
0.11 |
0.17 |
0.10 |
||
|
P2O25 |
4.68 |
13.00 |
5.07 |
0.44 |
1.90 |
1.30 |
||
|
SO3 |
0.12 |
0.10 |
0.36 |
0.25 |
1.93 |
0.95 |
There is a preponderance of alkali salts, which is problematic. Na and K from the soil nutrients find their way into the ash and they increase progressively as the fertilizer usage increases. These salts are low melting and highly scale forming on superheater (SH) tubes. The ash deformation temperatures are very low, causing serious problems of slagging and fouling. Not all the agrofuels have high alkalinity in ash, as for example, bagasse, rice husk, wood chips, bark, and so on. When high alkalinity is present in ash, the remedy lies in
• Lowering the FEGT below ~800°C (1560°F)
• Restricting the final SH temperature
• Minimizing the refractory in the furnace
• Adopting fully water-cooled walls in furnaces
• Increasing the residence times and lowering the volumetric heat release rates
• Preventing carry-over of ash and fuel from furnace by air curtain
• Provisioning generous and judicious soot blower (SB)
Such that the gases are cool enough to prevent ash melting and attaching to tubes. Even then an uninterrupted operation of the boiler may not be assured. Density of fuel ash is very low and the tendency to break down into very fine powder is very high, making dust collection rather difficult.



 19 августа, 2013
19 августа, 2013  admin
admin 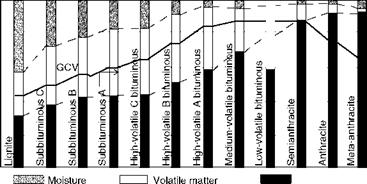
 Опубликовано в рубрике
Опубликовано в рубрике