The fuel burner is an important component of any boiler. Burner designs have undergone several iterations during the last decade. Burner suppliers such as Coen and Todd are offering burners that result in single-digit NOx emissions and very low CO levels, competing with the SCR system presently used in the industry for single-digit NOx emissions. However, these burners use a large amount of flue gas recirculation, and flame stability at low loads is a concern. Development work is going on to improve on these results. Fuel or air staging and
BOILER DUTY & EFFICIENCY CALCULATIONS
|
Boiler Parameters 1. steam press — psig = 400 2.steam temp — F = 750 3. sfeam flow — Lb/h = 100000 4. feed water temp — F = 320 5. blow down-% = 1. 6. process steam — Lb/h — |

|
1 boiler duty — MM Btu/h = 109-93 2.excess air — % — 18. 3. amb temp — F = 80 4. exit gas temp — F = 404 5. re! humidity — %- SO Flue Gas Analysis — % voi (wet /dry) |
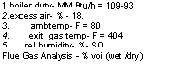 Combination firing — 2 fuels fired PROJECT sample units — British
Combination firing — 2 fuels fired PROJECT sample units — British
6. TOC o "1-5" h z со2 8.99 / 10.73
7.
|
[ gas flow, analysis, air flow, efficiencies shown here Pertain to effective average conditions if multiple fuels are fired..] |
![]() H2o 16.19
H2o 16.19
8. n2 71.92 / 85 81
9. o2 2.9 / 3.46
10. so2 . /
11. total air — Lb/h = 115402
12. totalflue gas — Lb/h -121312
13. efficiency-% HHV =82.7
14. efficiency-% LHV =90.71
15. adiab comb temp — F — 3207
Air heated by boiler flue gas ото airheater FUEL NO 1 gвs^Wvфоьiппл~T^~dьtr=7П]
Methane-97 ethane= 2 1
Fuel S. air input HHV — MM Btu/h = 95.54 fuel fired — Lb/h = 4021 ad comb temp — F = 3164 air/fuel ratio=20.S gas/fuel =215 eff-lhv -% =90 55 eff-hhv-% =81.69 tosses.’ drygas-%= 6.22 airmoisture= .17 fuel moisture= 11.12 radiation= .3 unaccounted= 5 flue gas analysis: % vol co2= 8.1 h2o= 17.8 n2= 71.22 o2= 2 89 so2=
LHV — Btu/lb = 21439 HHV — ВШЬ = 23764 fuel temp F = 80 air temp F = 80
NOx — ppmv/0.1 lb per MM Btu HHV = 83 1 CO — ppmv/0.1 lb CO per MM Btu HHV = 136.6
FUEL NO 2 oil-% weight [ % duty = 29]
Carbon — 87. hydrogen= 13. sulfur= . nitrogen=. oxygen= . deg API= 32 moisture^, fuel & air input HHV — MM Btu/h =37.38 fuel fired — Lb/h = 1895 ad comb temp — F = 3288 air/fuelratio=17.41 gas/fuel =18.41 eff-lhv-% =91.12 eff-hhv-% =85.28 losses: dry gas-%= 6.71 air moisture= 17 fuel moisture — 7 04 radiation — .3 unaccounted= .5 flue gas analysis: % voi co2- 11.29 h2o — 12.04 n2= 73.73 o2= 2.94 so2=
LHV-Btu/lb = 18463 HHV-Btu/lb = 19727 fuel temp F = 80 air temp F = 80
NOx-ppmv/0.1 lb per MM Btu HHV = 77.5 CO — ppmv/0 1 lb CO per MM Btu HHV = 127.4
Figure 3.14 Efficiency calculations for simultaneous firing of fuels.
Steam injection are the other methods used by burner suppliers to control NOx. Today single burners are used for capacities up to 300-350 MM Btu/h on gas or oil firing.
Often more than one fuel is fired in the burner. When different gaseous fuels are fired in a burner, the fuel gas pressure has to be adjusted at the burner inlet to ensure proper fuel flow.
Let us say that a burner is firing 5 MM Btu/h on LHV basis using a fuel of lower heating value, 1400Btu/ft3, and molecular weight 25.8 at a pressure of 30 psig. Assuming the nozzles remain the same, what should be done when a fuel of
heating value 700 Btu/ft3 whose molecular weight is 11.6 is fired, the duty being the same?
Solution: The gas pressure should be adjusted; otherwise it would be
Difficult to control the heat input. The pressure drop across the nozzles is related to the flow of fuel as follows (Subscripts 1 and 2 refer to fuels 1 and 2):
DP1 = KW2/MW = KQ2MW
Where Q = volumetric flow W = mass flow MW = molecular weight K is a constant = 30/Q, MW
Basically we are converting the pressure drop equation from mass to volumetric flow.
Because the heat input by both fuels is the same,
Q1 LHV! = Q2 LHV2
Where LHV is the lower heating value of the fuel, Btu/ft3.
TOC o "1-5" h z 30 2
Dp2 = ————- q2 mw2
2 q2 mw, Q2 2
Rewriting Q2 in terms of Q1 and simplifying, we have ™ f 7002 25.8 ,
Dp2=30* (us)xtls=17psi
Thus we should have a lower fuel gas pressure to ensure the same heat input.



 27 июля, 2013
27 июля, 2013  doctype
doctype  Опубликовано в рубрике
Опубликовано в рубрике