Good water chemistry is important for minimizing corrosion and the formation of scale in boilers. Steam-side cleanliness should be maintained in water tube as well as fire tube boilers. Plant engineers should do the following on a regular basis:
1. Maintain proper boiler water chemistry in the drum according to ABMA or ASME guidelines by using proper continuous blowdown rates. The calculation procedure for the blowdown rate based on feedwater and boiler water analysis is given in Q5.17.
2. Ensure that the feedwater analysis is fine and that there are no sudden changes in its conductivity or solids content.
3. Check steam purity to ensure that there are no sudden changes in its value. A sudden change may indicate carryover.
4. Watch superheated steam temperatures, particularly in boilers with large load swings. If slugs of water get carried into the steam during large load swings, the deposits are left behind after evaporation, potentially leading to tube failure. An indication of slugging, which is likely in boilers with small drums, is a sudden decrease in steam temperatures due to entrainment of water in the steam.
In the process of evaporating water to form steam, scale and sludge deposits form on the heated surfaces of a boiler tube. The chemical substances in the water concentrate in a film at the evaporation surface; the water displacing the bubbles of steam readily dissolves the soluble solids at the point of evaporation. Insoluble substances settle on the tube surfaces, forming a scale and leading to an increase in tube wall temperatures. Calcium bicarbonate, for example, decomposes in the boiler water to form calcium carbonate, carbon dioxide, and water. Calcium carbonate has limited solubility and will agglomerate at the heated surface to form a scale. Blowdown helps remove some of the deposits. Calcium sulfate is more soluble than calcium carbonate and will deposit as a heat-deterrent scale. Most scale-forming substances have a decreasing solubility in water with an increase in temperature.
In boilers that receive some hardness in the makeup water, deposits are generally compounds of calcium, sulfate, silica, magnesium, and phosphate. Depending on tube temperatures and heat flux and the solubility of these compounds as a function of temperature, these compounds can form deposits inside the boiler tubes. These scales, along with sludge and oils, form an insulating layer inside tubes at locations where the heat flux is intense. Alkalinity and pH of the water also affect the scale formation. Salts such as calcium sulfate and calcium phosphate deposit preferentially in hot regions. Boilers are considered generally clean if the deposits are less than 15 mg/cm2. Boilers having more than 40 mg/cm2 are considered very dirty. The least soluble compounds deposit first when boiling starts. Calcium carbonate deposits quickly, forming a white friable deposit. Magnesium phosphate is a binder that can produce very hard, adherent deposits. Insoluble silicates are present in many boilers. The presence of sodium hydroxide, phosphate, or sulfate may be considered proof that complete evaporation has occurred in the tubes, because these are easily soluble salts.
Sludge or easily removable deposits accumulate at the bottom of the tubes in the mud drum and should be removed by intermittent blowdown, generally once per shift. Based on conductivity readings, the frequency may be increased or decreased. Continuous blowdown is usually taken from the steam drum a few inches from above the waterline, where the concentration of solids is the highest.
Any boiler water treatment program should be reviewed with a water chemistry consultant, because this program can vary on a case-to-case basis. Generally the objective is to add chemicals to prevent scale formation caused by feedwater hardness constituents such as calcium and magnesium compounds and to provide pH control in the boiler to enhance maintenance of a protective oxide film on boiler water surfaces. There are methods such a phosphate-hydroxide, coordinated phosphate, chelant treatment, and polymer treatment methods. In medium and low pressure boilers, all these methods have been used.
Carryover of impurities with steam is a major concern in boilers having superheaters and also if steam is used in a steam turbine. Carryover results from both ineffective mechanical separation methods and vaporous carryover of certain salts. Vaporous carryover is a function of steam density and can be controlled only by controlling the boiler water solids, whereas mechanical carryover is governed by the efficiency of the steam separators used. Total solids carryover in steam is the sum of mechanical and vaporous carryover of impurities.
The steam purity requirements for saturated steam turbines are not stringent. Because the saturated steam begins to condense on the first stage of the turbine, water-soluble contaminants carried with the steam do not form deposits. Unless the steam is contaminated with solid particles or acidic gases, its purity does not significantly affect the turbine performance. However, there can be erosion concerns due to water droplets moving at high speeds.
With superheated steam, steam purity is critical to the turbine. Salts that are soluble in superheated steam may condense or precipitate and adhere to the metal surfaces as the steam is cooled when it expands. Deposition from steam can cause turbine valves to stick. Reduced efficiency and turbine imbalance are the other concerns. Deposition and corrosion occur in the ‘‘salt zone’’ just above the saturation line and on surfaces in the wet steam zone. The solubility of all low volatility impurities such as salts, hydroxides, silicon dioxide, and metal oxides decreases as steam expands in the turbine and is lowest at the saturation line. The moisture formed has the ability to dissolve most of the salts and carry them downstream. The critical region for deposition in turbines operating on superheated steam is the blade row located just upward of the Wilson line.
Mechanical carryover results from entrainment of small droplets of boiler water in the separated steam. Because the entrained water droplets contain the same concentration and proportions of solids as in the boiler water, the steam will also contain these solids as a function of its moisture content.
Foaming in the boiler water will also result in carryover. Common causes are excessive boiler water solids, excessive alkalinity, or the presence of organic matter such as oil. Continuous blowdown should be done to maintain the boiler water concentration below the ASME/ABMA levels.
Unlike mechanical carryover, vaporous carryover is selective because it depends on the solubility of the salts in steam. Silica is an example of a contaminant that has this tendency, particularly at high steam pressures, above 700psig. Boiler water of a higher pH helps minimize the carryover. Drum internals (Fig. 3.26) serve to remove moisture from the steam as it leaves the drum and enters the superheater. Generally the belly pan collects the steam-water mixture from the riser tubes and directs it inside the drum, where a chevron separator consisting of multiple vanes with tortuous paths separates the moisture from the steam. The mass flow of the mixture is the circulation ratio times the steam generation. Hence the belly pan width must be sized to handle the flow of this mixture. The steam purity required depends on the application. Saturated steam used in process heating applications can have a large carryover of solids, as much as 3-5 ppm. Drum internals need not be elaborate in these cases. A few steam turbine suppliers demand steam purity in the range of parts per billion for superheated steam, whereas some accept even 100 ppb total dissolved solids.
|
1 — steam-water mixture |
|
2 — wet steam 3 — dry steam 4 — drain pipe 5 — belly pan |
|
6 — steam drum 7 — chevron separator |
 |
|
|
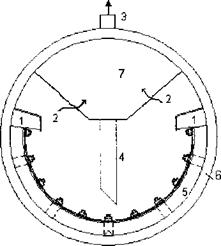
Restrictions are also placed on sodium and silica in steam. Typical silica levels are 20ppb. By maintaining proper boiler water chemistry as suggested in Q5.17, per ABMA and ASME, one can ensure that the steam purity is acceptable. Maintaining an alkaline condition (pH about 10-11.5) in the boiler water minimizes corrosion in the boiler; however, the alkalinity should also not exceed 700 ppm CaCO3. Above this level chemical reactions liberate CO2 into steam, which results in the corrosion of steam and return lines.
As far as the feedwater is concerned, proper deaeration and the removal of oxygen by chemical methods helps. Demineralized water is required if it is used for attemperation to control the steam temperature. Once-through steam generators and HRSGs need zero solids because complete evaporation of water occurs inside the tubes. Dissolved oxygen is the factor most responsible for the corrosion of steel surfaces in contact with water. Oxygen should be less than 5-7 ppb to minimize these concerns. Chemicals such as hydrazine or sodium sulfite are added to minimize oxygen corrosion.
Scale formation can affect the tube wall temperatures in fire tube as well as water tube boilers; as discussed above.
A few plants do not spend sufficient money on water treatment facilities. Table 3.9 shows how a large amount of blowdown increases the cost of operation and why it pays to invest in a good water treatment system. Corrosion and steam purity problems result in additional costs, which cannot be quantified because they lead to unscheduled maintenance. The additional amount of fuel fired to generate the same amount of steam is significant over a period of time. I have seen blowdown on the order of 15-20% in a few refineries.
Table 3.9 Cost of Blowdown3
|
2 |
![]()
|
2 |
![]()
Steam flow, lb/h Steam pressure, psig Steam temperature, °F Feedwater temperature, °F
Blowdown, %
Boiler duty, MM Btu/h Heat input, MM Btu/h Flash steam recovery, % Additional cost, $/y
1GG, GGG
3GG
Sat
23G
1G
1GG.8 1G2.4
121.5 123.4
2G 36,48G
1GG, GGG
85G
85G
23G
1G
123.1 125.7
148.4 151.5
33 49,85G
|
A |
![]() Boiler efficiency = 83% HHV; fuel cost = $3/MM Btu. Operating for 8000h/y.
Boiler efficiency = 83% HHV; fuel cost = $3/MM Btu. Operating for 8000h/y.



 2 августа, 2013
2 августа, 2013  doctype
doctype  Опубликовано в рубрике
Опубликовано в рубрике